EngA
- Description: GTPase essential for ribosome 50S subunit assembly
| Gene name | yphC |
| Synonyms | engA |
| Essential | yes PubMed |
| Product | GTPase |
| Function | ribosome assembly |
| Gene expression levels in SubtiExpress: engA | |
| MW, pI | 48 kDa, 5.248 |
| Gene length, protein length | 1308 bp, 436 aa |
| Immediate neighbours | gpsA, ypzH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 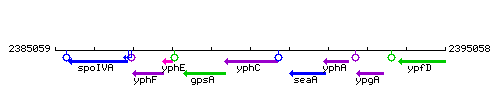
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
translation, essential genes, GTP-binding proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22840
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Binds and hydrolyzes GTP and readily exchanges GDP for GTP
- Protein family: EngA subfamily (according to Swiss-Prot) Era/Obg family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 2HJG (complex with GDP)
- UniProt: P50743
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- for expression, purification in E. coli with N-terminal His-tag, in pWH844: pGP846, available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Naotake Ogasawara, Nara, Japan
Your additional remarks
References
Reviews
Original publications
Anne-Emmanuelle Foucher, Jean-Baptiste Reiser, Christine Ebel, Dominique Housset, Jean-Michel Jault
Potassium acts as a GTPase-activating element on each nucleotide-binding domain of the essential Bacillus subtilis EngA.
PLoS One: 2012, 7(10);e46795
[PubMed:23056455]
[WorldCat.org]
[DOI]
(I p)
Laura Schaefer, William C Uicker, Catherine Wicker-Planquart, Anne-Emmanuelle Foucher, Jean-Michel Jault, Robert A Britton
Multiple GTPases participate in the assembly of the large ribosomal subunit in Bacillus subtilis.
J Bacteriol: 2006, 188(23);8252-8
[PubMed:16997968]
[WorldCat.org]
[DOI]
(P p)
Stephen P Muench, Ling Xu, Svetlana E Sedelnikova, David W Rice
The essential GTPase YphC displays a major domain rearrangement associated with nucleotide binding.
Proc Natl Acad Sci U S A: 2006, 103(33);12359-64
[PubMed:16894162]
[WorldCat.org]
[DOI]
(P p)
Ling Xu, Stephen P Muench, Anna Roujeinikova, Svetlana E Sedelnikova, David W Rice
Cloning, purification and preliminary crystallographic analysis of the Bacillus subtilis GTPase YphC-GDP complex.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2006, 62(Pt 5);435-7
[PubMed:16682769]
[WorldCat.org]
[DOI]
(I p)
Takuya Morimoto, Pek Chin Loh, Tomohiro Hirai, Kei Asai, Kazuo Kobayashi, Shigeki Moriya, Naotake Ogasawara
Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis.
Microbiology (Reading): 2002, 148(Pt 11);3539-3552
[PubMed:12427945]
[WorldCat.org]
[DOI]
(P p)