CsaA
- Description: molecular chaperone involved in protein secretion
| Gene name | csaA |
| Synonyms | |
| Essential | no |
| Product | molecular chaperone |
| Function | protein secretion |
| Gene expression levels in SubtiExpress: csaA | |
| Interactions involving this protein in SubtInteract: CsaA | |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 11 kDa, 8.691 |
| Gene length, protein length | 330 bp, 110 aa |
| Immediate neighbours | bsrF, yobQ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 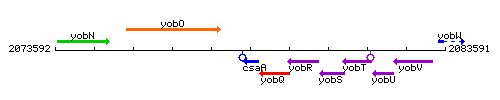
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed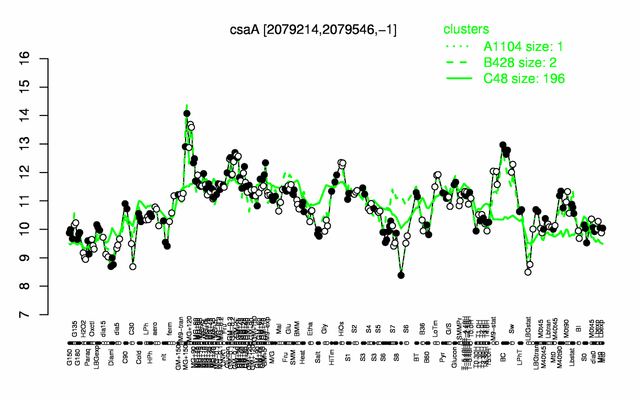
| |
Contents
Categories containing this gene/protein
protein secretion, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU19040
Phenotypes of a mutant
From unsuccessful trials to knock out the gene it was concluded that csaA apparently is essential. However, in a conditional mutant (Pspac-csaA), depletion of the gene product is not lethal. But secretion of some proteins including SdpC was reported to be affected unter such conditions.PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Secretion associated chaperone. Assumed functional analogue of E. coli SecB
- Protein family: FIG1 subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: CsaA contains a tRNA binding domain.
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure: 2NZO
- UniProt: P37584
- KEGG entry: [2]
- E.C. number:
Additional information
The functional entity of CsaA is the homodimer.
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: Pspac-csaA PubMed
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Yuliya A Shapova, Mark Paetzel
Crystallographic analysis of Bacillus subtilis CsaA.
Acta Crystallogr D Biol Crystallogr: 2007, 63(Pt 4);478-85
[PubMed:17372352]
[WorldCat.org]
[DOI]
(P p)
Dirk Linde, Rudolf Volkmer-Engert, Sandra Schreiber, Jörg P Müller
Interaction of the Bacillus subtilis chaperone CsaA with the secretory protein YvaY.
FEMS Microbiol Lett: 2003, 226(1);93-100
[PubMed:13129613]
[WorldCat.org]
[DOI]
(P p)
J P Müller, J Ozegowski, S Vettermann, J Swaving, K H Van Wely, A J Driessen
Interaction of Bacillus subtilis CsaA with SecA and precursor proteins.
Biochem J: 2000, 348 Pt 2(Pt 2);367-73
[PubMed:10816431]
[WorldCat.org]
(P p)
Jörg P Müller, Sierd Bron, Gerard Venema, Jan Maarten van Dijl
Chaperone-like activities of the CsaA protein of Bacillus subtilis.
Microbiology (Reading): 2000, 146 ( Pt 1);77-88
[PubMed:10658654]
[WorldCat.org]
[DOI]
(P p)