AhrC
Revision as of 08:06, 20 April 2012 by 134.76.70.252 (talk)
- Description: AhrC represses the genes for arginine biosynthesis and activates the genes for arginine catabolism.
| Gene name | ahrC |
| Synonyms | argR |
| Essential | no |
| Product | transcriptional regulator |
| Function | transcriptional regulator of arginine metabolic genes |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 16,7 kDa, 5.52 |
| Gene length, protein length | 447 bp, 149 amino acids |
| Immediate neighbours | recN, yqxC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 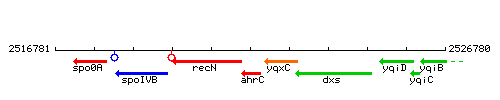
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, utilization of amino acids, transcription factors and their control
This gene is a member of the following regulons
The AhrC regulon
The gene
Basic information
- Locus tag: BSU24250
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcriptional activator/ repressor of genes involved in arginine metabolism
- Protein family: argR family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): L-arginine is the co-factor required for transcription repression/ activation
- Effectors of protein activity:
- Localization: Cytoplasm
Database entries
- Structure: 2P5M (C-Terminus), 2P5K (complex with an 18bp DNA operator), 2P5L (N-terminus), 1F9N, NCBI PubMed,N-Terminus NCBI, C-Terminus NCBI, complex with an 18bp DNA operator NCBI
- UniProt: P17893
- KEGG entry: [3]
Additional information
Expression and regulation
- Sigma factor:
- Additional information:
Biological materials
- Mutant: GP729 (aphA3), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Simon Phillips, Leeds University, UK Homepage
Michel Debarbouille, Pasteur Institute, Paris, France Homepage
Your additional remarks
References