BslA
- Description: bacterial hydrophobin, forms water-repellent surface layer of the biofilm, inhibitor of KinA autophosphorylation, and subsequently of entry into sporulation
| Gene name | bslA |
| Synonyms | yuaB, sivB |
| Essential | no |
| Product | biofilm surface layer, inhibitor of KinA autophosphorylation |
| Function | biofilm formation, control of entry into sporulation via the phosphorelay |
| Gene expression levels in SubtiExpress: bslA | |
| MW, pI | 19 kDa, 9.987 |
| Gene length, protein length | 543 bp, 181 aa |
| Immediate neighbours | gbsR, ktrA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 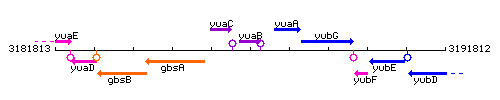
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed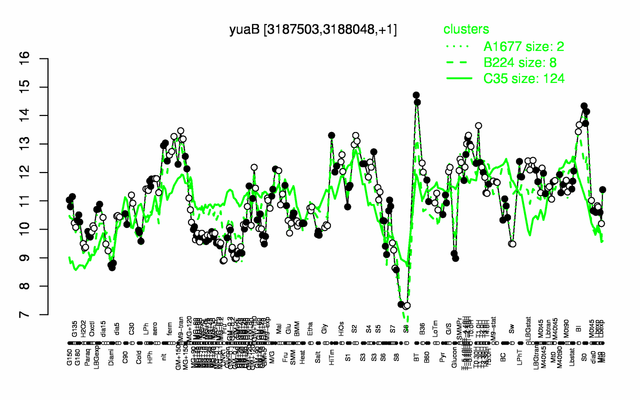
| |
Contents
Categories containing this gene/protein
biofilm formation, phosphorelay
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31080
Phenotypes of a mutant
Database entries
- BsubCyc: BSU31080
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Protein family:
- Paralogous protein(s): SivA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU31080
- UniProt: P71014
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- Physpank-bslA based on vector pDR111: pDRyuaB2 PubMed
- lacZ fusion:
- pNW500 PbslA-lacZ fusion in pDG1663 PubMed
- GFP fusion:
- PbslA-gfp fusion in pSG1151 vector: pSGyuaB PubMed
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Laura Hobley, Catriona Harkins, Cait E MacPhee, Nicola R Stanley-Wall
Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes.
FEMS Microbiol Rev: 2015, 39(5);649-69
[PubMed:25907113]
[WorldCat.org]
[DOI]
(I p)
Lynne S Cairns, Laura Hobley, Nicola R Stanley-Wall
Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms.
Mol Microbiol: 2014, 93(4);587-98
[PubMed:24988880]
[WorldCat.org]
[DOI]
(I p)
Ursula Hofer
Bacterial physiology: a raincoat for Bacillus subtilis.
Nat Rev Microbiol: 2013, 11(10);660-1
[PubMed:23979433]
[WorldCat.org]
[DOI]
(I p)
Akos T Kovács, Jordi van Gestel, Oscar P Kuipers
The protective layer of biofilm: a repellent function for a new class of amphiphilic proteins.
Mol Microbiol: 2012, 85(1);8-11
[PubMed:22607588]
[WorldCat.org]
[DOI]
(I p)
Original publications