TapA
Revision as of 11:12, 7 January 2014 by 134.76.70.252 (talk)
- Description: required for the anchoring of the TasA amyloid fibers to the cell and for the initiation of fiber polymerization, minor fiber component
| Gene name | tapA |
| Synonyms | yqhD, yqxM |
| Essential | no |
| Product | TasA anchoring/assembly protein |
| Function | biofilm formation |
| Gene expression levels in SubtiExpress: tapA | |
| Interactions involving this protein in SubtInteract: TapA | |
| Regulation of this protein in SubtiPathways: tapA | |
| MW, pI | 28 kDa, 6.677 |
| Gene length, protein length | 759 bp, 253 aa |
| Immediate neighbours | sipW, yqzG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 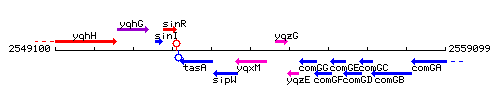
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed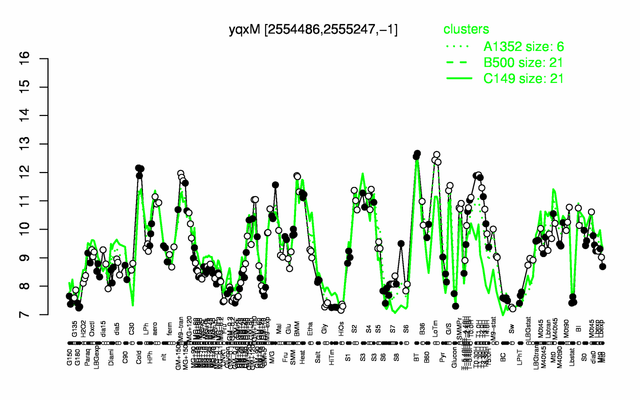
| |
Contents
Categories containing this gene/protein
biofilm formation, membrane proteins
This gene is a member of the following regulons
AbrB regulon, RemA regulon, SinR regulon
The gene
Basic information
- Locus tag: BSU24640
Phenotypes of a mutant
The mutants are able to form a biofilm in the presence of D-amino acids PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity: D-amino acids lead to disappearance of TapA from the cell wall PubMed
Database entries
- Structure:
- UniProt: P40949
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications