RefZ
- Description: regulator of FtsZ, facilitates switch from medial to polar FtsZ ring placement during sporulation
| Gene name | refZ |
| Synonyms | yttP |
| Essential | no |
| Product | regulator of FtsZ |
| Function | relocalization of the FtsZ ring |
| Interactions involving this protein in SubtInteract: RefZ | |
| MW, pI | 24 kDa, 6.706 |
| Gene length, protein length | 621 bp, 207 aa |
| Immediate neighbours | hisJ, ytsP |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 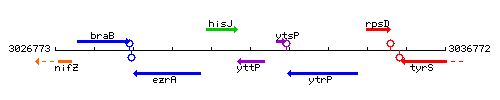
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed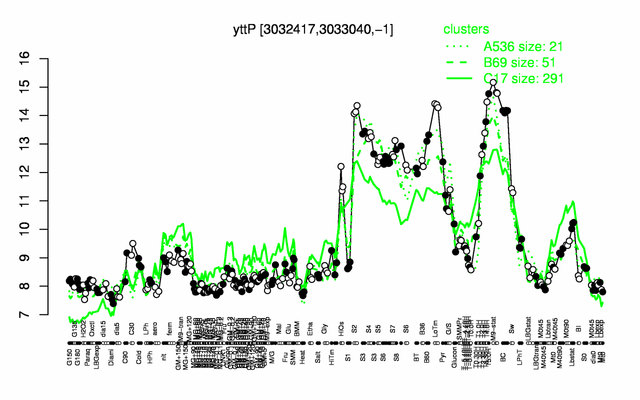
| |
Contents
Categories containing this gene/protein
cell division, sporulation/ other
This gene is a member of the following regulons
CcpA regulon, PhoP regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU29630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: facilitates switch from medial to polar FtsZ ring placement during sporulation PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- TetR-like DNA-binding helix-turn-helix domain at the N-terminus PubMed
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- polar foci PubMed
Database entries
- Structure:
- UniProt: O34970
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: refZ PubMed
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jennifer K Wagner-Herman, Remi Bernard, Roisin Dunne, Alexandre W Bisson-Filho, Krithika Kumar, Trang Nguyen, Lawrence Mulcahy, John Koullias, Frederico J Gueiros-Filho, David Z Rudner
RefZ facilitates the switch from medial to polar division during spore formation in Bacillus subtilis.
J Bacteriol: 2012, 194(17);4608-18
[PubMed:22730127]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Zoltán Prágai, Nicholas E E Allenby, Nicola O'Connor, Sarah Dubrac, Georges Rapoport, Tarek Msadek, Colin R Harwood
Transcriptional regulation of the phoPR operon in Bacillus subtilis.
J Bacteriol: 2004, 186(4);1182-90
[PubMed:14762014]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)