MalL
- Description: alpha-glucosidase
| Gene name | malL |
| Synonyms | yvdL |
| Essential | no |
| Product | alpha-glucosidase |
| Function | starch and maltodextrin utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism, Sugar catabolism | |
| MW, pI | 65 kDa, 4.98 |
| Gene length, protein length | 1683 bp, 561 aa |
| Immediate neighbours | pgcM, yvdK |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 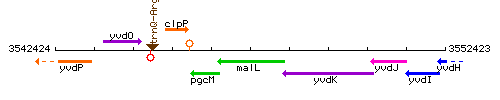
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU34560
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of (1->6)-alpha-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by alpha-amylase, and in isomaltose (according to Swiss-Prot)
- Protein family: glycosyl hydrolase 13 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: O06994
- KEGG entry: [2]
- E.C. number: 3.2.1.10
Additional information
Expression and regulation
- Regulation:
- induced in the presence of maltose PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Stefan Schönert, Sabine Seitz, Holger Krafft, Eva-Anne Feuerbaum, Iris Andernach, Gabriele Witz, Michael K Dahl
Maltose and maltodextrin utilization by Bacillus subtilis.
J Bacteriol: 2006, 188(11);3911-22
[PubMed:16707683]
[WorldCat.org]
[DOI]
(P p)
S Schönert, T Buder, M K Dahl
Properties of maltose-inducible alpha-glucosidase MalL (sucrase-isomaltase-maltase) in Bacillus subtilis: evidence for its contribution to maltodextrin utilization.
Res Microbiol: 1999, 150(3);167-77
[PubMed:10229946]
[WorldCat.org]
[DOI]
(P p)
S Schönert, T Buder, M K Dahl
Identification and enzymatic characterization of the maltose-inducible alpha-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis.
J Bacteriol: 1998, 180(9);2574-8
[PubMed:9573215]
[WorldCat.org]
[DOI]
(P p)