LeuC
- Description: 3-isopropylmalate dehydratase (large subunit)
| Gene name | leuC |
| Synonyms | |
| Essential | no |
| Product | 3-isopropylmalate dehydratase (large subunit) |
| Function | biosynthesis of leucine |
| MW, pI | 52 kDa, 6.127 |
| Gene length, protein length | 1416 bp, 472 aa |
| Immediate neighbours | leuD, leuB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 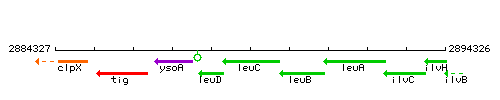
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28260
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: (2R,3S)-3-isopropylmalate = (2S)-2-isopropylmaleate + H2O (according to Swiss-Prot)
- Protein family: LeuC type 1 subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P80858
- KEGG entry: [3]
- E.C. number: 4.2.1.33
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation:
- repressed in the absence of good nitrogen sources (glutamine or ammonium) (TnrA) PubMed
- repressed during growth in the presence of branched chain amino acids (CodY) PubMed
- repressed by casamino acids PubMed
- expressed in the absence of branched-chain amino acids (BCAA)
- expression is stimulated in the presence of glucose PubMed
- repressed by CodY PubMed
- less expressed under conditions of extreme iron limitation (FsrA) PubMed
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Mäder et al. (2002) Transcriptome and Proteome Analysis of Bacillus subtilis Gene Expression Modulated by Amino Acid Availability. J. Bacteriol 184: 1844288-4295 PubMed
- Gerth et al. (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190:321-331 PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed