Zur
Revision as of 09:44, 17 April 2014 by 134.76.70.252 (talk)
- Description: transcriptional repressor, regulates zinc homeostasis
| Gene name | zur |
| Synonyms | yqfV |
| Essential | no |
| Product | transcriptional repressor (Fur family) |
| Function | regulation of zinc homeostasis (yciC, znuA-znuC-znuB) |
| Gene expression levels in SubtiExpress: zur | |
| Regulatory function of this protein in SubtiPathways: zur | |
| Metabolic function and regulation of this protein in SubtiPathways: Zur | |
| MW, pI | 16 kDa, 4.856 |
| Gene length, protein length | 435 bp, 145 aa |
| Immediate neighbours | yqfW, yqfU |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 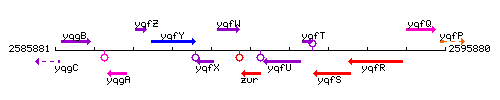
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed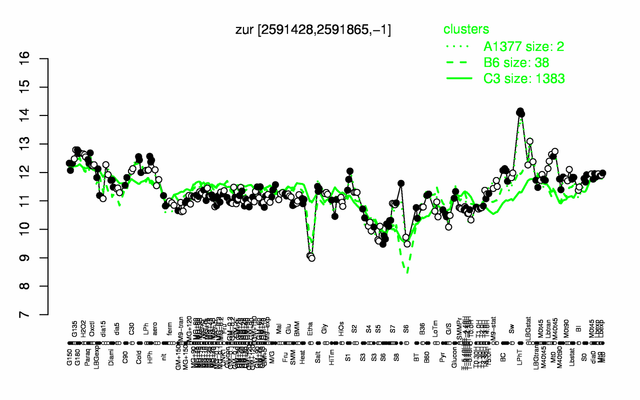
| |
Contents
Categories containing this gene/protein
trace metal homeostasis (Cu, Zn, Ni, Mn, Mo), transcription factors and their control, phosphoproteins
This gene is a member of the following regulons
The Zur regulon
The gene
Basic information
- Locus tag: BSU25100
Phenotypes of a mutant
Database entries
- BsubCyc: BSU25100
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Fur family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated on ser/ thr/ tyr PubMed
- Cofactor(s):
- Effectors of protein activity:
- sequential binding of zinc (two atoms per monomer) results in the activation of Zur and thus in repression of the genes of the Zur regulon PubMed
- Interactions:
- forms dimers PubMed
Database entries
- BsubCyc: BSU25100
- Structure:
- UniProt: P54479
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References