SunA
- Description: sublancin 168 lantibiotic antimicrobial precursor peptide
| Gene name | sunA |
| Synonyms | yolG |
| Essential | no |
| Product | sublancin 168 lantibiotic antimicrobial precursor peptide |
| Function | antimicrobial peptide |
| Gene expression levels in SubtiExpress: sunA | |
| MW, pI | 5 kDa, 7.963 |
| Gene length, protein length | 168 bp, 56 aa |
| Immediate neighbours | sunT, sunI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 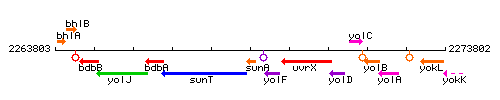
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds, toxins, antitoxins and immunity against toxins, SP-beta prophage, membrane proteins
This gene is a member of the following regulons
Abh regulon, AbrB regulon, Rok regulon, YvrHb regulon
The gene
Basic information
- Locus tag: BSU21480
Phenotypes of a mutant
Database entries
- BsubCyc: BSU21480
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: IPP isomerase type 2 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: contains a glucose attached to a cysteine residue, glycosylation is essential for its antimicrobial activity PubMed
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU21480
- UniProt: P68577
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: sunA PubMed
- Regulatory mechanism:
- Additional information: the mRNA is very stable (half-life > 15 min) PubMed
Biological materials
- Mutant:
- GP1563 (aphA3), available in Jörg Stülke's lab
- GP1565 (sunA-sunI, aphA3), available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Shengyue Ji, Weili Li, Abdul Rasheed Baloch, Meng Wang, Binyun Cao
Improved production of sublancin via introduction of three characteristic promoters into operon clusters responsible for this novel distinct glycopeptide biosynthesis.
Microb Cell Fact: 2015, 14;17
[PubMed:25879813]
[WorldCat.org]
[DOI]
(I e)
Chantal V Garcia De Gonzalo, Lingyang Zhu, Trent J Oman, Wilfred A van der Donk
NMR structure of the S-linked glycopeptide sublancin 168.
ACS Chem Biol: 2014, 9(3);796-801
[PubMed:24405370]
[WorldCat.org]
[DOI]
(I p)
Rebecca Mendez, Alba Gutierrez, Jasmin Reyes, Leticia Márquez-Magaña
The extracytoplasmic function sigma factor SigY is important for efficient maintenance of the Spβ prophage that encodes sublancin in Bacillus subtilis.
DNA Cell Biol: 2012, 31(6);946-55
[PubMed:22400495]
[WorldCat.org]
[DOI]
(I p)
Huan Wang, Wilfred A van der Donk
Substrate selectivity of the sublancin S-glycosyltransferase.
J Am Chem Soc: 2011, 133(41);16394-7
[PubMed:21910430]
[WorldCat.org]
[DOI]
(I p)
Trent J Oman, John M Boettcher, Huan Wang, Xenia N Okalibe, Wilfred A van der Donk
Sublancin is not a lantibiotic but an S-linked glycopeptide.
Nat Chem Biol: 2011, 7(2);78-80
[PubMed:21196935]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Yun Luo, John D Helmann
Extracytoplasmic function sigma factors with overlapping promoter specificity regulate sublancin production in Bacillus subtilis.
J Bacteriol: 2009, 191(15);4951-8
[PubMed:19465659]
[WorldCat.org]
[DOI]
(I p)
Mark A Strauch, Benjamin G Bobay, John Cavanagh, Fude Yao, Angelo Wilson, Yoann Le Breton
Abh and AbrB control of Bacillus subtilis antimicrobial gene expression.
J Bacteriol: 2007, 189(21);7720-32
[PubMed:17720793]
[WorldCat.org]
[DOI]
(P p)
Masakuni Serizawa, Keisuke Kodama, Hiroki Yamamoto, Kazuo Kobayashi, Naotake Ogasawara, Junichi Sekiguchi
Functional analysis of the YvrGHb two-component system of Bacillus subtilis: identification of the regulated genes by DNA microarray and northern blot analyses.
Biosci Biotechnol Biochem: 2005, 69(11);2155-69
[PubMed:16306698]
[WorldCat.org]
[DOI]
(P p)
Mark Albano, Wiep Klaas Smits, Linh T Y Ho, Barbara Kraigher, Ines Mandic-Mulec, Oscar P Kuipers, David Dubnau
The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions.
J Bacteriol: 2005, 187(6);2010-9
[PubMed:15743949]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
S H Paik, A Chakicherla, J N Hansen
Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168.
J Biol Chem: 1998, 273(36);23134-42
[PubMed:9722542]
[WorldCat.org]
[DOI]
(P p)