HtrA
- Description: membrane-anchored protein quality control protease, serine protease Do
| Gene name | htrA |
| Synonyms | ykdA |
| Essential | no |
| Product | serine protease Do |
| Function | protein quality control |
| Gene expression levels in SubtiExpress: htrA | |
| Metabolic function and regulation of this protein in SubtiPathways: htrA | |
| MW, pI | 47 kDa, 4.699 |
| Gene length, protein length | 1347 bp, 449 aa |
| Immediate neighbours | ykcC, proG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 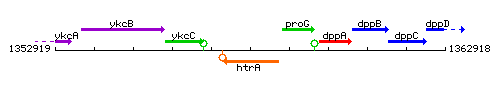
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
proteolysis, heat shock proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU12900
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family: PDZ (DHR) domain (according to Swiss-Prot)
- Paralogous protein(s): HtrC
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O34358
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: htrA (according to DBTBS)
- Regulation:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Ross E Dalbey, Peng Wang, Jan Maarten van Dijl
Membrane proteases in the bacterial protein secretion and quality control pathway.
Microbiol Mol Biol Rev: 2012, 76(2);311-30
[PubMed:22688815]
[WorldCat.org]
[DOI]
(I p)
Tim Clausen, Markus Kaiser, Robert Huber, Michael Ehrmann
HTRA proteases: regulated proteolysis in protein quality control.
Nat Rev Mol Cell Biol: 2011, 12(3);152-62
[PubMed:21326199]
[WorldCat.org]
[DOI]
(I p)
Original publications