SunA
- Description: sublancin 168 lantibiotic antimicrobial precursor peptide
| Gene name | sunA |
| Synonyms | yolG |
| Essential | no |
| Product | sublancin 168 lantibiotic antimicrobial precursor peptide |
| Function | antimicrobial peptide |
| Gene expression levels in SubtiExpress: sunA | |
| MW, pI | 5 kDa, 7.963 |
| Gene length, protein length | 168 bp, 56 aa |
| Immediate neighbours | sunT, sunI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 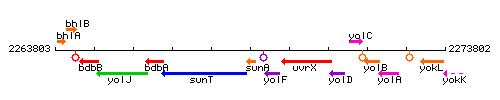
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds, toxins, antitoxins and immunity against toxins, SP-beta prophage, membrane proteins
This gene is a member of the following regulons
Abh regulon, AbrB regulon, Rok regulon, YvrHb regulon
The gene
Basic information
- Locus tag: BSU21480
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: IPP isomerase type 2 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: contains a glucose attached to a cysteine residue, glycosylation is essential for its antimicrobial activity PubMed
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- UniProt: P68577
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: sunA PubMed
- Regulatory mechanism:
- Additional information: the mRNA is very stable (half-life > 15 min) PubMed
Biological materials
- Mutant: GP1563 (aphA3), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Chantal V Garcia De Gonzalo, Lingyang Zhu, Trent J Oman, Wilfred A van der Donk
NMR structure of the S-linked glycopeptide sublancin 168.
ACS Chem Biol: 2014, 9(3);796-801
[PubMed:24405370]
[WorldCat.org]
[DOI]
(I p)
Rebecca Mendez, Alba Gutierrez, Jasmin Reyes, Leticia Márquez-Magaña
The extracytoplasmic function sigma factor SigY is important for efficient maintenance of the Spβ prophage that encodes sublancin in Bacillus subtilis.
DNA Cell Biol: 2012, 31(6);946-55
[PubMed:22400495]
[WorldCat.org]
[DOI]
(I p)
Huan Wang, Wilfred A van der Donk
Substrate selectivity of the sublancin S-glycosyltransferase.
J Am Chem Soc: 2011, 133(41);16394-7
[PubMed:21910430]
[WorldCat.org]
[DOI]
(I p)
Trent J Oman, John M Boettcher, Huan Wang, Xenia N Okalibe, Wilfred A van der Donk
Sublancin is not a lantibiotic but an S-linked glycopeptide.
Nat Chem Biol: 2011, 7(2);78-80
[PubMed:21196935]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Yun Luo, John D Helmann
Extracytoplasmic function sigma factors with overlapping promoter specificity regulate sublancin production in Bacillus subtilis.
J Bacteriol: 2009, 191(15);4951-8
[PubMed:19465659]
[WorldCat.org]
[DOI]
(I p)
Mark A Strauch, Benjamin G Bobay, John Cavanagh, Fude Yao, Angelo Wilson, Yoann Le Breton
Abh and AbrB control of Bacillus subtilis antimicrobial gene expression.
J Bacteriol: 2007, 189(21);7720-32
[PubMed:17720793]
[WorldCat.org]
[DOI]
(P p)
Masakuni Serizawa, Keisuke Kodama, Hiroki Yamamoto, Kazuo Kobayashi, Naotake Ogasawara, Junichi Sekiguchi
Functional analysis of the YvrGHb two-component system of Bacillus subtilis: identification of the regulated genes by DNA microarray and northern blot analyses.
Biosci Biotechnol Biochem: 2005, 69(11);2155-69
[PubMed:16306698]
[WorldCat.org]
[DOI]
(P p)
Mark Albano, Wiep Klaas Smits, Linh T Y Ho, Barbara Kraigher, Ines Mandic-Mulec, Oscar P Kuipers, David Dubnau
The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions.
J Bacteriol: 2005, 187(6);2010-9
[PubMed:15743949]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
S H Paik, A Chakicherla, J N Hansen
Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168.
J Biol Chem: 1998, 273(36);23134-42
[PubMed:9722542]
[WorldCat.org]
[DOI]
(P p)