Aag
- Description: general stress protein, similar to DNA-3-methyladenine glycosidase II, required for protection against paraquat stress
| Gene name | aag |
| Synonyms | yxlJ |
| Essential | no |
| Product | unknown |
| Function | survival of stress conditions |
| Gene expression levels in SubtiExpress: aag | |
| MW, pI | 21 kDa, 6.117 |
| Gene length, protein length | 588 bp, 196 aa |
| Immediate neighbours | yxzF, katX |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 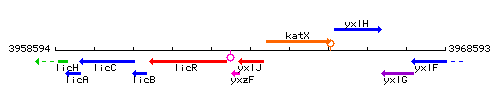
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed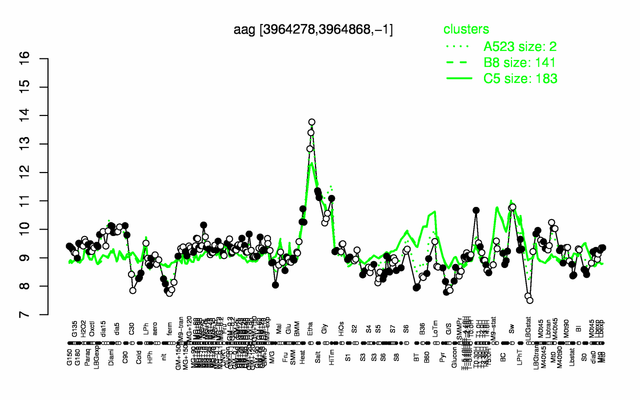
| |
Contents
Categories containing this gene/protein
DNA repair/ recombination, general stress proteins (controlled by SigB), resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU38620
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: removes hypoxanthine, 3-alkylated purines, and 1,N6-ethenoadenine from DNA; hypoxanthine and 1,N6-ethenoadenine are the preferred substrates PubMed
- Protein family: DNA glycosylase MPG family (according to Swiss-Prot) AAG family of 3-methyladenine DNA glycosylases
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P94378
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Alexander Reder, Dirk Höper, Ulf Gerth, Michael Hecker
Contributions of individual σB-dependent general stress genes to oxidative stress resistance of Bacillus subtilis.
J Bacteriol: 2012, 194(14);3601-10
[PubMed:22582280]
[WorldCat.org]
[DOI]
(I p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
Randi M Aamodt, Pål Ø Falnes, Rune F Johansen, Erling Seeberg, Magnar Bjørås
The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates.
J Biol Chem: 2004, 279(14);13601-6
[PubMed:14729667]
[WorldCat.org]
[DOI]
(P p)