CopZ
Revision as of 09:10, 23 April 2012 by 134.76.70.252 (talk)
- Description: copper transport protein, metallochaperone
| Gene name | copZ |
| Synonyms | yvgY |
| Essential | no |
| Product | copper transport protein, metallochaperone |
| Function | resistance to copper |
| Interactions involving this protein in SubtInteract: CopZ | |
| Metabolic function and regulation of this protein in SubtiPathways: metal ion homeostasis | |
| MW, pI | 7 kDa, 4.162 |
| Gene length, protein length | 207 bp, 69 aa |
| Immediate neighbours | copA, csoR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 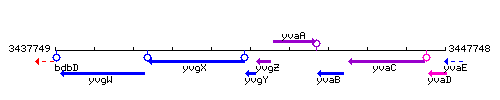
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transporters/ other, trace metal homeostasis (Cu, Zn, Ni, Mn, Mo), resistance against toxic metals
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33510
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): carries a tetranuclear Cu(I) cluster (as [Cu4(S-Cys)4(N-His)2] cluster) PubMed
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 2QIF
- UniProt: O32221
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Reviews
Amie K Boal, Amy C Rosenzweig
Structural biology of copper trafficking.
Chem Rev: 2009, 109(10);4760-79
[PubMed:19824702]
[WorldCat.org]
[DOI]
(I p)
Original publications