YlxP
- Description: general stress protein
| Gene name | ylxP |
| Synonyms | ymxD |
| Essential | no |
| Product | unknown |
| Function | unknown |
| MW, pI | 10 kDa, 7.895 |
| Gene length, protein length | 276 bp, 92 aa |
| Immediate neighbours | infB, rbfA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 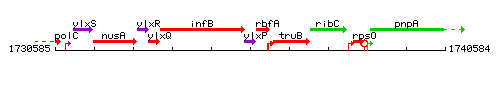
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
general stress proteins (controlled by SigB)
This gene is a member of the following regulons
SigB regulon, stringent response
The gene
Basic information
- Locus tag: BSU16640
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P32730
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
K Shazand, J Tucker, M Grunberg-Manago, J C Rabinowitz, T Leighton
Similar organization of the nusA-infB operon in Bacillus subtilis and Escherichia coli.
J Bacteriol: 1993, 175(10);2880-7
[PubMed:8491709]
[WorldCat.org]
[DOI]
(P p)