Difference between revisions of "AccA"
(→Reviews) |
(→Biological materials) |
||
| Line 133: | Line 133: | ||
* '''Expression vector:''' | * '''Expression vector:''' | ||
| + | ** purification from ''B. subtilis'' with an C-terminal Strep-tag, for [[SPINE]], (in [[pGP382]]): pGP1723, available in [[Jörg Stülke]]'s lab | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| Line 138: | Line 139: | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' ''B. pertussis'' adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Jörg Stülke]]'s lab |
| + | |||
| + | * '''FLAG-tag construct:''' | ||
| + | ** GP1487 (''spc'', based on [[pGP1331]]), available in [[Jörg Stülke]]'s lab | ||
* '''Antibody:''' | * '''Antibody:''' | ||
Revision as of 10:34, 6 March 2015
- Description: acetyl-CoA carboxylase (alpha subunit)
| Gene name | accA |
| Synonyms | |
| Essential | yes PubMed |
| Product | acetyl-CoA carboxylase (alpha subunit)) |
| Function | production of malonyl-CoA, the substrate for fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: accA | |
| Interactions involving this protein in SubtInteract: AccA | |
| Metabolic function and regulation of this protein in SubtiPathways: accA | |
| MW, pI | 36 kDa, 6.087 |
| Gene length, protein length | 975 bp, 325 aa |
| Immediate neighbours | pfkA, accD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 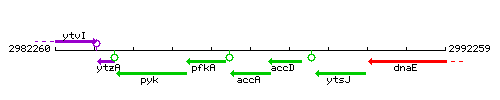
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29200
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU29200
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + acetyl-CoA + HCO3- = ADP + phosphate + malonyl-CoA (according to Swiss-Prot)
- Protein family: accA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot), Membrane-proximal (Spotty) PubMed
Database entries
- BsubCyc: BSU29200
- UniProt: O34847
- KEGG entry: [3]
- E.C. number: 6.4.1.2
Additional information
Expression and regulation
- Sigma factor:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1047 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 795 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 910 PubMed
Biological materials
- Mutant:
- Expression vector:
- purification from B. subtilis with an C-terminal Strep-tag, for SPINE, (in pGP382): pGP1723, available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct:
- GP1487 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original Publications