Difference between revisions of "CotA"
(→References) |
(→Original publications) |
||
| Line 142: | Line 142: | ||
<pubmed>23202530</pubmed> | <pubmed>23202530</pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>15699190,3135411,11514528,1518043,2821284, 19933362, 20200715 20551082 20822511 21369750 22281748 23859715 22171814 22410485 15383836 24293734 24733162 </pubmed> | + | <pubmed>15699190,3135411,11514528,1518043,2821284, 19933362, 20200715 20551082 20822511 21369750 22281748 23859715 22171814 22410485 15383836 24293734 24733162 25259857 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:31, 29 September 2014
- Description: laccase, bilirubin oxidase, spore coat protein (outer)
| Gene name | cotA |
| Synonyms | pig |
| Essential | no |
| Product | laccase, bilirubin oxidase |
| Function | resistance of the spore |
| Gene expression levels in SubtiExpress: cotA | |
| MW, pI | 58 kDa, 5.89 |
| Gene length, protein length | 1539 bp, 513 aa |
| Immediate neighbours | yeaA, gabP |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 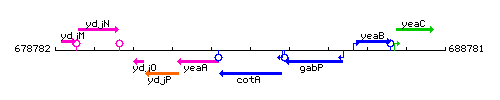
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed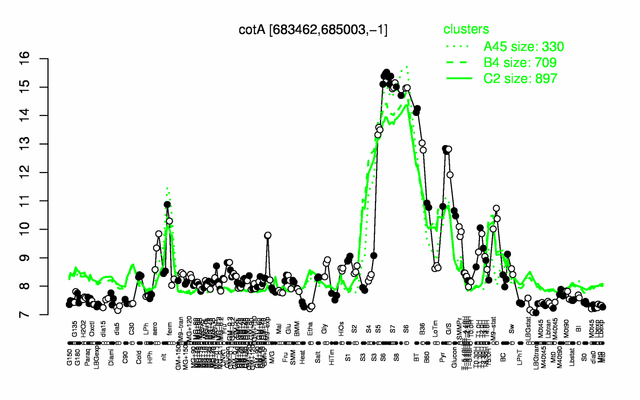
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
GerE regulon, SigK regulon, Efp-dependent proteins
The gene
Basic information
- Locus tag: BSU06300
Phenotypes of a mutant
Database entries
- BsubCyc: BSU06300
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU06300
- Structure: 2BHF (reduced form)
- UniProt: P07788
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: cotA (according to DBTBS)
- Additional information:
- translation is likely to require Efp due to the presence of several consecutive proline residues PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications