Difference between revisions of "MntR"
| Line 64: | Line 64: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU24520&redirect=T BSU24520] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 99: | Line 100: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU24520&redirect=T BSU24520] | ||
* '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=2F5D 2F5D] (complex with manganese), [http://www.rcsb.org/pdb/explore.do?structureId=2HYG 2HYG] (apo-form) | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=2F5D 2F5D] (complex with manganese), [http://www.rcsb.org/pdb/explore.do?structureId=2HYG 2HYG] (apo-form) | ||
Revision as of 14:11, 2 April 2014
- Description: transcriptional regulator, (repression of mntH and mntA-mntB-mntC-mntD under high Mn(II) conditions)
| Gene name | mntR |
| Synonyms | yqhN |
| Essential | no |
| Product | transcriptional regulator (DtxR family) |
| Function | regulation of manganese transport |
| Gene expression levels in SubtiExpress: mntR | |
| Metabolic function and regulation of this protein in SubtiPathways: mntR | |
| MW, pI | 16 kDa, 5.631 |
| Gene length, protein length | 426 bp, 142 aa |
| Immediate neighbours | yqhO, lipM |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 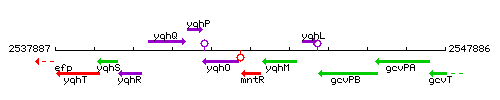
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed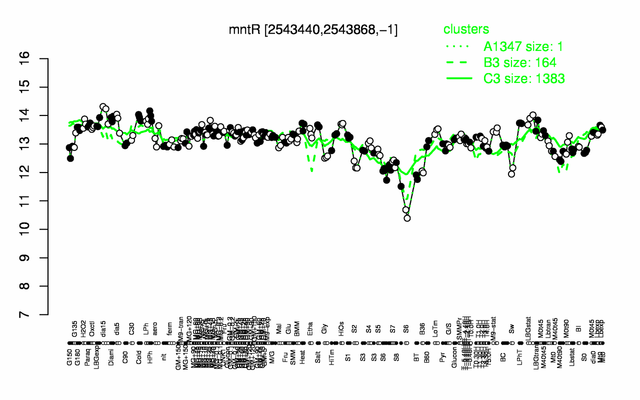
| |
Contents
Categories containing this gene/protein
trace metal homeostasis (Cu, Zn, Ni, Mn, Mo), transcription factors and their control, membrane proteins
This gene is a member of the following regulons
The MntR regulon:
The gene
Basic information
- Locus tag: BSU24520
Phenotypes of a mutant
Database entries
- BsubCyc: BSU24520
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Mn(2+) acts as co-repressor (according to PubMed)
- Effectors of protein activity:
- Interactions:
- active as dimer (according to PubMed)
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU24520
- UniProt: P54512
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Richard Brennan, Houston, Texas, USA Homepage
Your additional remarks
References
Reviews
Original Publications
Amanda M McGuire, Bonnie J Cuthbert, Zhen Ma, Kristen D Grauer-Gray, Megan Brunjes Brophy, Kayce A Spear, Sumarin Soonsanga, Joseph I Kliegman, Sarah L Griner, John D Helmann, Arthur Glasfeld
Roles of the A and C sites in the manganese-specific activation of MntR.
Biochemistry: 2013, 52(4);701-13
[PubMed:23298157]
[WorldCat.org]
[DOI]
(I p)
Misha V Golynskiy, William A Gunderson, Michael P Hendrich, Seth M Cohen
Metal binding studies and EPR spectroscopy of the manganese transport regulator MntR.
Biochemistry: 2006, 45(51);15359-72
[PubMed:17176058]
[WorldCat.org]
[DOI]
(I p)
Mark A DeWitt, Joseph I Kliegman, John D Helmann, Richard G Brennan, David L Farrens, Arthur Glasfeld
The conformations of the manganese transport regulator of Bacillus subtilis in its metal-free state.
J Mol Biol: 2007, 365(5);1257-65
[PubMed:17118401]
[WorldCat.org]
[DOI]
(P p)
Joseph I Kliegman, Sarah L Griner, John D Helmann, Richard G Brennan, Arthur Glasfeld
Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis.
Biochemistry: 2006, 45(11);3493-505
[PubMed:16533030]
[WorldCat.org]
[DOI]
(P p)
Misha V Golynskiy, Talib C Davis, John D Helmann, Seth M Cohen
Metal-induced structural organization and stabilization of the metalloregulatory protein MntR.
Biochemistry: 2005, 44(9);3380-9
[PubMed:15736948]
[WorldCat.org]
[DOI]
(P p)
Scot A Lieser, Talib C Davis, John D Helmann, Seth M Cohen
DNA-binding and oligomerization studies of the manganese(II) metalloregulatory protein MntR from Bacillus subtilis.
Biochemistry: 2003, 42(43);12634-42
[PubMed:14580210]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, Charles M Moore, Qiang Que, Tao Wang, Rick W Ye, John D Helmann
The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons.
Mol Microbiol: 2003, 49(6);1477-91
[PubMed:12950915]
[WorldCat.org]
[DOI]
(P p)
Arthur Glasfeld, Emmanuel Guedon, John D Helmann, Richard G Brennan
Structure of the manganese-bound manganese transport regulator of Bacillus subtilis.
Nat Struct Biol: 2003, 10(8);652-7
[PubMed:12847518]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, John D Helmann
Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators.
Mol Microbiol: 2003, 48(2);495-506
[PubMed:12675807]
[WorldCat.org]
[DOI]
(P p)
Q Que, J D Helmann
Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins.
Mol Microbiol: 2000, 35(6);1454-68
[PubMed:10760146]
[WorldCat.org]
[DOI]
(P p)