Difference between revisions of "YwaD"
(→References) |
|||
| Line 132: | Line 132: | ||
=References= | =References= | ||
| − | <pubmed>15668014,23426795,18957862, 23787698 </pubmed> | + | <pubmed>15668014,23426795,18957862, 23787698 24633010 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:39, 19 March 2014
- Description: double-zinc aminopeptidase
| Gene name | ywaD |
| Synonyms | ipa-8r |
| Essential | no |
| Product | double-zinc aminopeptidase |
| Function | unknown |
| Gene expression levels in SubtiExpress: ywaD | |
| MW, pI | 49 kDa, 6.638 |
| Gene length, protein length | 1365 bp, 455 aa |
| Immediate neighbours | tyrZ, sasA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 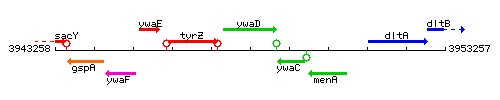
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed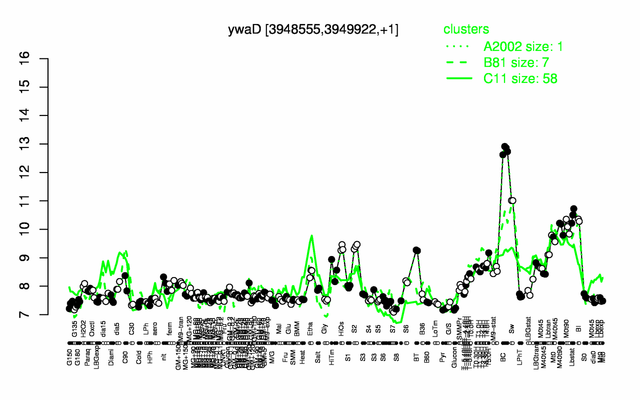
| |
Contents
Categories containing this gene/protein
poorly characterized/ putative enzymes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU38470
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: M28B subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization:
- extracellular (signal peptide) PubMed
Database entries
- Structure:
- UniProt: P25152
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: ywaD PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References