Difference between revisions of "NrgB"
(→Original publications) |
Czschiedrich (talk | contribs) |
||
| Line 26: | Line 26: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[nrgA]]'', ''[[bcrC]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[nrgA]]'', ''[[bcrC]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''[http:// | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU36520 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU36520 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU36520 DNA_with_flanks] |
| − | |||
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:nrgB_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:nrgB_context.gif]] | ||
Revision as of 09:54, 11 November 2013
- Description: nitrogen-regulated PII-like protein
| Gene name | nrgB |
| Synonyms | glnK |
| Essential | no |
| Product | nitrogen-regulated PII-like protein |
| Function | regulation of ammonium uptake |
| Gene expression levels in SubtiExpress: nrgB | |
| Interactions involving this protein in SubtInteract: NrgB | |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 12 kDa, 8.825 |
| Gene length, protein length | 348 bp, 116 aa |
| Immediate neighbours | nrgA, bcrC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 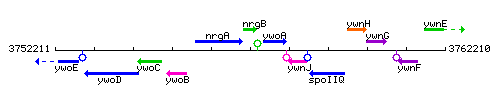
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, glutamate metabolism, transcription factors and their control, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36520
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: P(II) protein family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane, via NrgA PubMed
Database entries
- UniProt: Q07428
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant: GP259 (cat), GP255 (nrgAB, cat), both available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Jörg Stülke lab
Labs working on this gene/protein
Susan Fisher, Boston, USA homepage
Your additional remarks
References
Reviews
Original publications