Difference between revisions of "AtpD"
| Line 1: | Line 1: | ||
| − | * '''Description:''' ATP synthase (subunit beta) <br/><br/> | + | * '''Description:''' [[ATP synthase]], part of the F1 complex (subunit beta) <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || ATP synthase (subunit beta)) | + | |style="background:#ABCDEF;" align="center"| '''Product''' || [[ATP synthase]] (subunit beta)) |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || ATP synthesis | + | |style="background:#ABCDEF;" align="center"|'''Function''' || [[ATP synthesis]] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU36810 atpD] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU36810 atpD] | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/AtpD AtpD] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 51 kDa, 4.611 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 51 kDa, 4.611 | ||
| Line 35: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 91: | Line 89: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| + | ** [[AtpB|A]](1)-[[AtpF|B]](2)-[[AtpE|C]](12) - [[AtpA|alpha]](3)-[[AtpD|beta]](3)-[[AtpG|gamma]]-[[AtpH|delta]]-[[AtpC|epsilon]] | ||
| − | * '''[[Localization]]:''' | + | * '''[[Localization]]:''' |
| + | ** membrane [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | ||
| + | ** peripheral via theF0 complex | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/101/motm.do?momID=72&evtc=Suggest&evta=Moleculeof%20the%20Month&evtl=OtherOptions see here an overview on ATPase structure] |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P37809 P37809] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P37809 P37809] | ||
| Line 112: | Line 113: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=atpD_3781491_3782912_-1 atpD] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=atpD_3781491_3782912_-1 atpD] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 140: | Line 141: | ||
=References= | =References= | ||
| − | + | == Reviews == | |
| + | <pubmed> 23356252 23341301, 23267178 22822068 21524994 19489730 17208001 16730323 </pubmed> | ||
| + | == Original publications == | ||
<pubmed>7961438,,16493705 18763711, </pubmed> | <pubmed>7961438,,16493705 18763711, </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:00, 11 August 2013
- Description: ATP synthase, part of the F1 complex (subunit beta)
| Gene name | atpD |
| Synonyms | |
| Essential | no |
| Product | ATP synthase (subunit beta)) |
| Function | ATP synthesis |
| Gene expression levels in SubtiExpress: atpD | |
| Interactions involving this protein in SubtInteract: AtpD | |
| MW, pI | 51 kDa, 4.611 |
| Gene length, protein length | 1419 bp, 473 aa |
| Immediate neighbours | atpC, atpG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 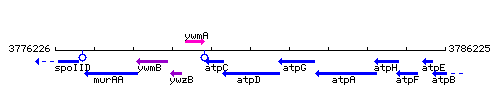
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed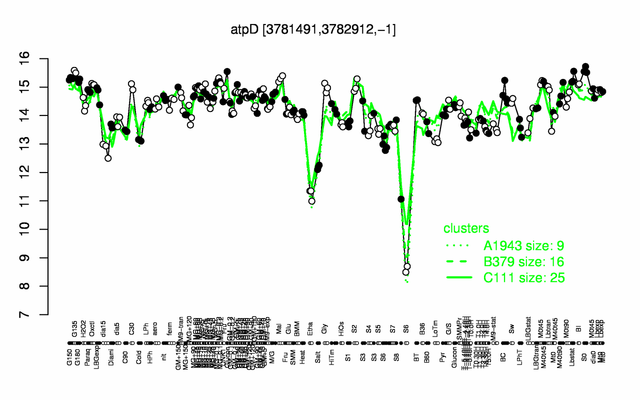
| |
Contents
Categories containing this gene/protein
ATP synthesis, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36810
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + H2O + H+(In) = ADP + phosphate + H+(Out) (according to Swiss-Prot), ATP synthesis see a video
- Protein family: ATPase alpha/beta chains family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated on ser/ thr/ tyr PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane PubMed
- peripheral via theF0 complex
Database entries
- Structure: see here an overview on ATPase structure
- UniProt: P37809
- KEGG entry: [3]
- E.C. number: 3.6.3.14
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications