Difference between revisions of "BslA"
(→Database entries) |
|||
| Line 95: | Line 95: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' ** [http://www.rcsb.org/pdb/explore.do?structureId=4BHU 4BHU] {{PubMed|23904481}} | + | * '''Structure:''' |
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=4BHU 4BHU] {{PubMed|23904481}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P71014 P71014] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P71014 P71014] | ||
Revision as of 07:57, 2 August 2013
- Description: bacterial hydrophobin, forms water-repellent surface layer of the biofilm, inhibitor of KinA autophosphorylation, and subsequently of entry into sporulation
| Gene name | bslA |
| Synonyms | yuaB, sivB |
| Essential | no |
| Product | biofilm surface layer, inhibitor of KinA autophosphorylation |
| Function | biofilm formation, control of entry into sporulation via the phosphorelay |
| Gene expression levels in SubtiExpress: bslA | |
| MW, pI | 19 kDa, 9.987 |
| Gene length, protein length | 543 bp, 181 aa |
| Immediate neighbours | gbsR, ktrA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 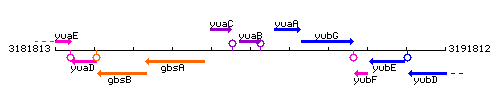
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed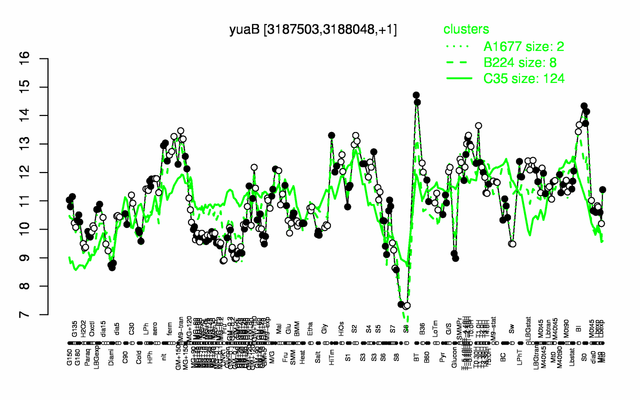
| |
Contents
Categories containing this gene/protein
biofilm formation, phosphorelay
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31080
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Protein family:
- Paralogous protein(s): SivA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P71014
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- Physpank-yuaB based on vector pDR111: pDRyuaB2 PubMed
- lacZ fusion:
- pNW500 PyuaB-lacZ fusion in pDG1663 PubMed
- GFP fusion:
- PyuaB-gfp fusion in pSG1151 vector: pSGyuaB PubMed
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Akos T Kovács, Jordi van Gestel, Oscar P Kuipers
The protective layer of biofilm: a repellent function for a new class of amphiphilic proteins.
Mol Microbiol: 2012, 85(1);8-11
[PubMed:22607588]
[WorldCat.org]
[DOI]
(I p)
Original publications