Difference between revisions of "RnjB"
(→References) |
|||
| Line 139: | Line 139: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | + | <pubmed>20458164 21893280 23403287,21957024 22568516 </pubmed> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | <pubmed>20458164 21893280</pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | + | <pubmed>15831787 18204464 18713320 19193632, 19633085 20025672 21862575 21893285</pubmed> | |
| − | <pubmed>15831787 18204464 18713320 19193632, 19633085 20025672 21862575 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:21, 13 July 2013
- Description: RNase J2
| Gene name | rnjB |
| Synonyms | ymfA |
| Essential | no |
| Product | RNase J2 |
| Function | RNA processing and degradation |
| Gene expression levels in SubtiExpress: rnjB | |
| Interactions involving this protein in SubtInteract: RNase J2 | |
| MW, pI | 56 kDa, 9.18 |
| Gene length, protein length | 1545 bp, 515 aa |
| Immediate neighbours | dapA, tepA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 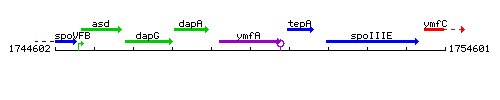
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed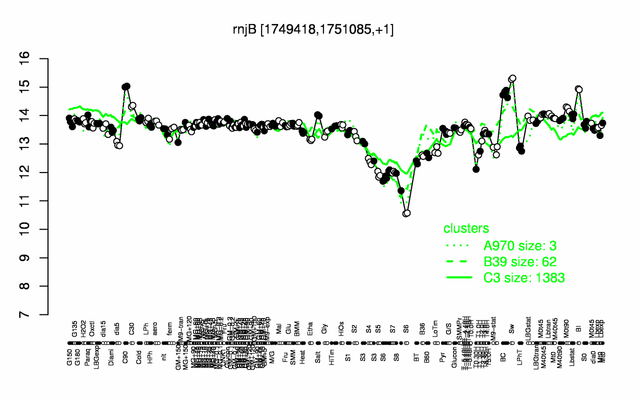
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16780
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: endoribonuclease, involved in processing of thrS mRNA
- Protein family: RNase J subfamily (according to Swiss-Prot)
- Paralogous protein(s): RnjA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- associated to the RNA degradosome PubMed
- RnjA-RnjB PubMed
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP45 (spc), GP1113 (miniTn10 spc), both available in Stülke lab
- Expression vector:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Harald Putzer, IBPC Paris, France Homepage
Your additional remarks
References
Reviews
Zbigniew Dominski, Agamemnon J Carpousis, Béatrice Clouet-d'Orval
Emergence of the β-CASP ribonucleases: highly conserved and ubiquitous metallo-enzymes involved in messenger RNA maturation and degradation.
Biochim Biophys Acta: 2013, 1829(6-7);532-51
[PubMed:23403287]
[WorldCat.org]
[DOI]
(P p)
Martin Lehnik-Habrink, Richard J Lewis, Ulrike Mäder, Jörg Stülke
RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases.
Mol Microbiol: 2012, 84(6);1005-17
[PubMed:22568516]
[WorldCat.org]
[DOI]
(I p)
David H Bechhofer
Bacillus subtilis mRNA decay: new parts in the toolkit.
Wiley Interdiscip Rev RNA: 2011, 2(3);387-94
[PubMed:21957024]
[WorldCat.org]
[DOI]
(I p)
Jamie Richards, Joel G Belasco
Ribonuclease J: how to lead a double life.
Structure: 2011, 19(9);1201-3
[PubMed:21893280]
[WorldCat.org]
[DOI]
(I p)
Ciarán Condon
What is the role of RNase J in mRNA turnover?
RNA Biol: 2010, 7(3);316-21
[PubMed:20458164]
[WorldCat.org]
[DOI]
(I p)
Original publications
Joseph A Newman, Lorraine Hewitt, Cecilia Rodrigues, Alexandra Solovyova, Colin R Harwood, Richard J Lewis
Unusual, dual endo- and exonuclease activity in the degradosome explained by crystal structure analysis of RNase J1.
Structure: 2011, 19(9);1241-51
[PubMed:21893285]
[WorldCat.org]
[DOI]
(I p)
Gintaras Deikus, David H Bechhofer
5' End-independent RNase J1 endonuclease cleavage of Bacillus subtilis model RNA.
J Biol Chem: 2011, 286(40);34932-40
[PubMed:21862575]
[WorldCat.org]
[DOI]
(I p)
Nathalie Mathy, Agnès Hébert, Peggy Mervelet, Lionel Bénard, Audrey Dorléans, Inés Li de la Sierra-Gallay, Philippe Noirot, Harald Putzer, Ciarán Condon
Bacillus subtilis ribonucleases J1 and J2 form a complex with altered enzyme behaviour.
Mol Microbiol: 2010, 75(2);489-98
[PubMed:20025672]
[WorldCat.org]
[DOI]
(I p)
Shiyi Yao, David H Bechhofer
Processing and stability of inducibly expressed rpsO mRNA derivatives in Bacillus subtilis.
J Bacteriol: 2009, 191(18);5680-9
[PubMed:19633085]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Fabian M Rothe, Christina Herzberg, Eva Wagner, Daniel Hellwig, Martin Lehnik-Habrink, Elke Hammer, Uwe Völker, Jörg Stülke
Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing.
Mol Cell Proteomics: 2009, 8(6);1350-60
[PubMed:19193632]
[WorldCat.org]
[DOI]
(I p)
Ulrike Mäder, Léna Zig, Julia Kretschmer, Georg Homuth, Harald Putzer
mRNA processing by RNases J1 and J2 affects Bacillus subtilis gene expression on a global scale.
Mol Microbiol: 2008, 70(1);183-96
[PubMed:18713320]
[WorldCat.org]
[DOI]
(I p)
Inés Li de la Sierra-Gallay, Léna Zig, Ailar Jamalli, Harald Putzer
Structural insights into the dual activity of RNase J.
Nat Struct Mol Biol: 2008, 15(2);206-12
[PubMed:18204464]
[WorldCat.org]
[DOI]
(I p)
Sergine Even, Olivier Pellegrini, Lena Zig, Valerie Labas, Joelle Vinh, Dominique Bréchemmier-Baey, Harald Putzer
Ribonucleases J1 and J2: two novel endoribonucleases in B.subtilis with functional homology to E.coli RNase E.
Nucleic Acids Res: 2005, 33(7);2141-52
[PubMed:15831787]
[WorldCat.org]
[DOI]
(I e)