Difference between revisions of "RpsN"
| Line 112: | Line 112: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rpsNA_141757_141942_1 rpsN] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rpsNA_141757_141942_1 rpsN] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
** [[SigA]] two promoters, about 140bp and 200bp upstream of the start codon {{PubMed|9371452}} | ** [[SigA]] two promoters, about 140bp and 200bp upstream of the start codon {{PubMed|9371452}} | ||
| Line 141: | Line 141: | ||
=References= | =References= | ||
| − | + | <pubmed>17163968,8635744,9371452, 19648245 ,19653700 20208344 11948165 22517742 23002217</pubmed> | |
| − | <pubmed>17163968,8635744,9371452, 19648245 ,19653700 20208344 11948165 22517742</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:31, 19 June 2013
- Description: ribosomal protein
| Gene name | rpsN |
| Synonyms | |
| Essential | yes PubMed |
| Product | ribosomal protein S14 |
| Function | translation |
| Gene expression levels in SubtiExpress: rpsN | |
| Interactions involving this protein in SubtInteract: RpsN | |
| MW, pI | 7 kDa, 10.914 |
| Gene length, protein length | 183 bp, 61 aa |
| Immediate neighbours | rplE, rpsH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 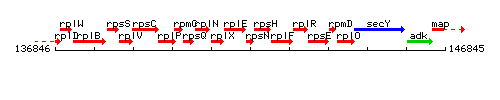
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
translation, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01290
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein S14P family (according to Swiss-Prot) Zinc-binding S14P subfamily (according to Swiss-Prot)
- Paralogous protein(s): YhzA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-41 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P12878
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon: rpsJ-rplC-rplD-rplW-rplB-rpsS-rplV-rpsC-rplP-rpmC-rpsQ-rplN-rplX-rplE-rpsN-rpsH-rplF-rplR-rpsE-rpmD-rplO PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References