Difference between revisions of "CtsR"
| Line 31: | Line 31: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ctsR_101449_101913_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:ctsR_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ctsR_101449_101913_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:ctsR_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU00830]] |
|- | |- | ||
|} | |} | ||
Revision as of 12:20, 16 May 2013
| Gene name | ctsR |
| Synonyms | yacG |
| Essential | no |
| Product | transcription repressor |
| Function | regulation of protein degradation |
| Gene expression levels in SubtiExpress: ctsR | |
| Interactions involving this protein in SubtInteract: CtsR | |
| Regulatory function and regulation of this protein in SubtiPathways: Stress, Phosphorelay | |
| MW, pI | 17 kDa, 9.261 |
| Gene length, protein length | 462 bp, 154 aa |
| Immediate neighbours | rrnW-5S, mcsA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 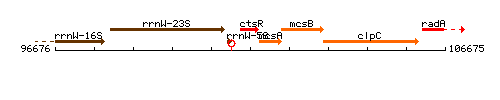
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed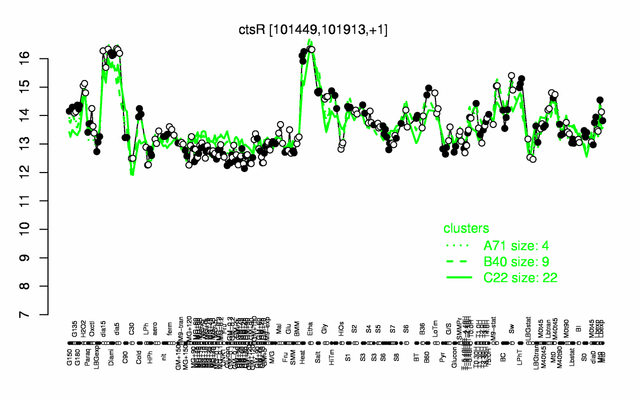
| |
Contents
Categories containing this gene/protein
proteolysis, transcription factors and their control, general stress proteins (controlled by SigB), heat shock proteins, phosphoproteins
This gene is a member of the following regulons
The CtsR regulon
The gene
Basic information
- Locus tag: BSU00830
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ctsR family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P37568
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information: the mRNA is very stable (half-life > 15 min) PubMed
Biological materials
- Mutant: ctsR::aphA3 availbale from the Gerth lab
ctsRG65P::spec available from the Gerth lab
- Expression vector: for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Gerth lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Gerth lab
Labs working on this gene/protein
Your additional remarks
References
Reviews
Additional reviews: PubMed
Original publications
Additional publications: PubMed
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Liang Tao, Partho Chattoraj, Indranil Biswas
CtsR regulation in mcsAB-deficient Gram-positive bacteria.
J Bacteriol: 2012, 194(6);1361-8
[PubMed:22247503]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kristina Hempel, Dierk-Christoph Pöther, Dörte Becher, Michael Hecker, Ulf Gerth
CtsR inactivation during thiol-specific stress in low GC, Gram+ bacteria.
Mol Microbiol: 2011, 79(3);772-85
[PubMed:21208299]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Stephan Michalik, Daniela Zühlke, Michael Hecker, Ulf Gerth
CtsR, the Gram-positive master regulator of protein quality control, feels the heat.
EMBO J: 2010, 29(21);3621-9
[PubMed:20852588]
[WorldCat.org]
[DOI]
(I p)
Marcus Miethke, Michael Hecker, Ulf Gerth
Involvement of Bacillus subtilis ClpE in CtsR degradation and protein quality control.
J Bacteriol: 2006, 188(13);4610-9
[PubMed:16788169]
[WorldCat.org]
[DOI]
(P p)
Pekka Varmanen, Finn K Vogensen, Karin Hammer, Airi Palva, Hanne Ingmer
ClpE from Lactococcus lactis promotes repression of CtsR-dependent gene expression.
J Bacteriol: 2003, 185(17);5117-24
[PubMed:12923084]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
J D Helmann, M F Wu, P A Kobel, F J Gamo, M Wilson, M M Morshedi, M Navre, C Paddon
Global transcriptional response of Bacillus subtilis to heat shock.
J Bacteriol: 2001, 183(24);7318-28
[PubMed:11717291]
[WorldCat.org]
[DOI]
(P p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
E Krüger, D Zühlke, E Witt, H Ludwig, M Hecker
Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor.
EMBO J: 2001, 20(4);852-63
[PubMed:11179229]
[WorldCat.org]
[DOI]
(P p)
I Derré, G Rapoport, T Msadek
The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37 degrees C.
Mol Microbiol: 2000, 38(2);335-47
[PubMed:11069659]
[WorldCat.org]
[DOI]
(P p)
I Derré, G Rapoport, T Msadek
CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria.
Mol Microbiol: 1999, 31(1);117-31
[PubMed:9987115]
[WorldCat.org]
[DOI]
(P p)
E Krüger, M Hecker
The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes.
J Bacteriol: 1998, 180(24);6681-8
[PubMed:9852015]
[WorldCat.org]
[DOI]
(P p)
E Krüger, T Msadek, M Hecker
Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon.
Mol Microbiol: 1996, 20(4);713-23
[PubMed:8793870]
[WorldCat.org]
[DOI]
(P p)
E Krüger, U Völker, M Hecker
Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance.
J Bacteriol: 1994, 176(11);3360-7
[PubMed:8195092]
[WorldCat.org]
[DOI]
(P p)