Difference between revisions of "MurAB"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || peptidoglycan precursor biosynthesis | |style="background:#ABCDEF;" align="center"|'''Function''' || peptidoglycan precursor biosynthesis | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU37100 murAB] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/cellwall.html Cell wall]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/cellwall.html Cell wall]''' | ||
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glpX]]'', ''[[ywjH]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glpX]]'', ''[[ywjH]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU37100 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU37100 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU37100 Advanced_DNA] |
|- | |- | ||
|- | |- | ||
Revision as of 14:05, 13 May 2013
- Description: UDP-N-acetylglucosamine 1-carboxyvinyltransferase
| Gene name | murAB |
| Synonyms | murZ, lssF, rev-4 |
| Essential | no |
| Product | UDP-N-acetylglucosamine 1-carboxyvinyltransferase |
| Function | peptidoglycan precursor biosynthesis |
| Gene expression levels in SubtiExpress: murAB | |
| Metabolic function and regulation of this protein in SubtiPathways: Cell wall | |
| MW, pI | 45 kDa, 5.831 |
| Gene length, protein length | 1287 bp, 429 aa |
| Immediate neighbours | glpX, ywjH |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 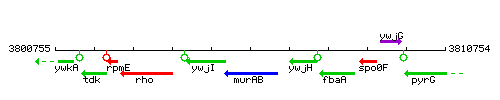
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU37100
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Phosphoenolpyruvate + UDP-N-acetyl-D-glucosamine = phosphate + UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine (according to Swiss-Prot)
- Protein family: MurA subfamily (according to Swiss-Prot)
- Paralogous protein(s): MurAA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 3SG1 (from B. anthracis, 50% identity, 81% similarity)
- UniProt: P19670
- KEGG entry: [2]
- E.C. number: 2.5.1.7
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutive PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Matthieu Jules, Ludovic Le Chat, Stéphane Aymerich, Dominique Le Coq
The Bacillus subtilis ywjI (glpX) gene encodes a class II fructose-1,6-bisphosphatase, functionally equivalent to the class III Fbp enzyme.
J Bacteriol: 2009, 191(9);3168-71
[PubMed:19270101]
[WorldCat.org]
[DOI]
(I p)