Difference between revisions of "Fmt"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || formylation of Met-tRNA(fMet) | |style="background:#ABCDEF;" align="center"|'''Function''' || formylation of Met-tRNA(fMet) | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU15730 fmt] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/tRNA_charging.html tRNA charging]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/tRNA_charging.html tRNA charging]''' | ||
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[def]]'', ''[[yloM]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[def]]'', ''[[yloM]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU15730 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU15730 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU15730 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:fmt_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:fmt_context.gif]] | ||
Revision as of 12:48, 13 May 2013
- Description: methionyl-tRNA formyltransferase
| Gene name | fmt |
| Synonyms | yloL |
| Essential | yes PubMed |
| Product | methionyl-tRNA formyltransferase |
| Function | formylation of Met-tRNA(fMet) |
| Gene expression levels in SubtiExpress: fmt | |
| Metabolic function and regulation of this protein in SubtiPathways: tRNA charging | |
| MW, pI | 34 kDa, 5.618 |
| Gene length, protein length | 951 bp, 317 aa |
| Immediate neighbours | def, yloM |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 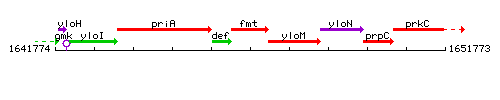
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed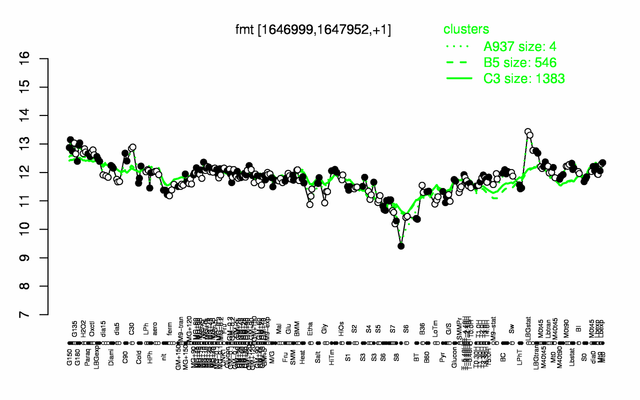
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15730
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 10-formyltetrahydrofolate + L-methionyl-tRNA(fMet) + H2O = tetrahydrofolate + N-formylmethionyl-tRNA(fMet) (according to Swiss-Prot)
- Protein family: fmt family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 3RFO (from Bacillus anthracis, 69% identity, 89% similarity)
- UniProt: P94463
- KEGG entry: [2]
- E.C. number: 2.1.2.9
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- translation is likely to require Efp due to the presence of several consecutive proline residues PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional references: PubMed
Yann Duroc, Carmela Giglione, Thierry Meinnel
Mutations in three distinct loci cause resistance to peptide deformylase inhibitors in Bacillus subtilis.
Antimicrob Agents Chemother: 2009, 53(4);1673-8
[PubMed:19171795]
[WorldCat.org]
[DOI]
(I p)
E Schmitt, S Blanquet, Y Mechulam
Structure of crystalline Escherichia coli methionyl-tRNA(f)Met formyltransferase: comparison with glycinamide ribonucleotide formyltransferase.
EMBO J: 1996, 15(17);4749-58
[PubMed:8887566]
[WorldCat.org]
(P p)