Difference between revisions of "PerR"
(→Original Publications) |
|||
| Line 144: | Line 144: | ||
==Original Publications== | ==Original Publications== | ||
| − | + | <pubmed> 8932315,18487332,14563870, 16766519,17158660,12486061,11532148,12180919,16166527, 15231799, 12029044,10913706, 21398634, 9701813, 16541078, 12029044, ,11532148, 12029044, 19508285 , 22194458 23645680 23057863</pubmed> | |
| − | <pubmed> 8932315,18487332,14563870, 16766519,17158660,12486061,11532148,12180919,16166527, 15231799, 12029044,10913706, 21398634, 9701813, 16541078, 12029044, ,11532148, 12029044, 19508285 , 22194458 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:35, 7 May 2013
- Description: transcriptional repressor of the peroxide regulon
| Gene name | perR |
| Synonyms | ygaG |
| Essential | no |
| Product | transcriptional repressor (Fur family) |
| Function | regulation of the response to peroxide |
| Gene expression levels in SubtiExpress: perR | |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 16 kDa, 5.888 |
| Gene length, protein length | 435 bp, 145 aa |
| Immediate neighbours | ygaF, ygzB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 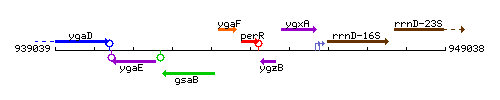
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The PerR regulon
The gene
Basic information
- Locus tag: BSU08730
Phenotypes of a mutant
- resistant to hydrogen peroxide, accumulates a porphyrin-like compound, and grows very slowly (due to heme sequestration by KatA) PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Fur family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- selective metal catalyzed oxidation of two histidine residues of the regulatory site results in induction (loss of DNA-binding activity) PubMed
- Cofactor(s): contains an Fe(2+) at the regulatory site and Zn(2+) PubMed
- Effectors of protein activity:
- responds to the presence of hydrogen peroxide
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P71086
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: perR PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant: HB0509 (spc), available in John Helmann's and Jörg Stülke's labs, also GP868 (fur::mls, perR::spc).
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Reviews
Original Publications