Difference between revisions of "PonA"
| Line 35: | Line 35: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 117: | Line 113: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ponA_2341444_2344188_1 ponA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ponA_2341444_2344188_1 ponA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] {{PubMed|22211522}}, [[SigM]] {{PubMed|18179421}} | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|22211522}}, [[SigM]] {{PubMed|18179421}} |
* '''Regulation:''' constitutive during vegetative growth [http://www.ncbi.nlm.nih.gov/sites/entrez/15758244 PubMed] | * '''Regulation:''' constitutive during vegetative growth [http://www.ncbi.nlm.nih.gov/sites/entrez/15758244 PubMed] | ||
| Line 147: | Line 143: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | + | <pubmed>21371139 </pubmed> | |
==Original publications== | ==Original publications== | ||
| − | <pubmed>8606187,15262952,9721295,10322023,19192185,7814321,18363795,14731276, 21320184,18763711 19192185, 18179421 </pubmed> | + | <pubmed>8606187,15262952,9721295,10322023,19192185,7814321,18363795,14731276, 21320184,18763711 19192185, 18179421 23531131 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:33, 28 March 2013
- Description: penicillin-binding protein 1A/1B
| Gene name | ponA |
| Synonyms | |
| Essential | no |
| Product | penicillin-binding protein 1A/1B |
| Function | bifunctional glucosyltransferase/ transpeptidase |
| Gene expression levels in SubtiExpress: ponA | |
| MW, pI | 99 kDa, 4.752 |
| Gene length, protein length | 2742 bp, 914 aa |
| Immediate neighbours | recU, ypoC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 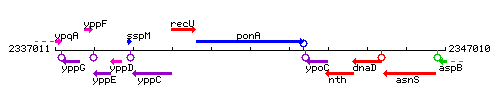
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall synthesis, cell envelope stress proteins (controlled by SigM, V, W, X, Y), membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22320
Phenotypes of a mutant
- prevents bulging of the cells when grown at low Mg(2+) concentrations, suppresses the lethal effect of a mreB mutation PubMed
- deletion of ponA restores growth and normal shape of a yvcK mutant on gluconeogenic carbon sources PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P39793
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation: constitutive during vegetative growth PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jeff Errington lab
- Antibody:
Labs working on this gene/protein
Jeff Errington, Newcastle University, UK homepage
Your additional remarks
References
Reviews
Original publications