Difference between revisions of "BcaP"
Raphael2215 (talk | contribs) |
|||
| Line 1: | Line 1: | ||
| − | + | * '''Description:''' branched-chain amino acid transporter <br/><br/> | |
| − | |||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
Revision as of 10:37, 10 August 2012
- Description: branched-chain amino acid transporter
| Gene name | bcaP |
| Synonyms | yhdG |
| Essential | no |
| Product | branched-chain amino acid transporter |
| Function | biosynthesis/acquisition of branched-chain amino acids |
| Gene expression levels in SubtiExpress: bcaP | |
| MW, pI | 49 kDa, 9.735 |
| Gene length, protein length | 1395 bp, 465 aa |
| Immediate neighbours | yhdF, yhdH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 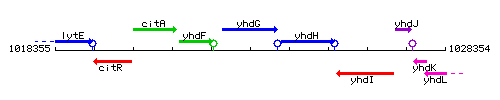
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed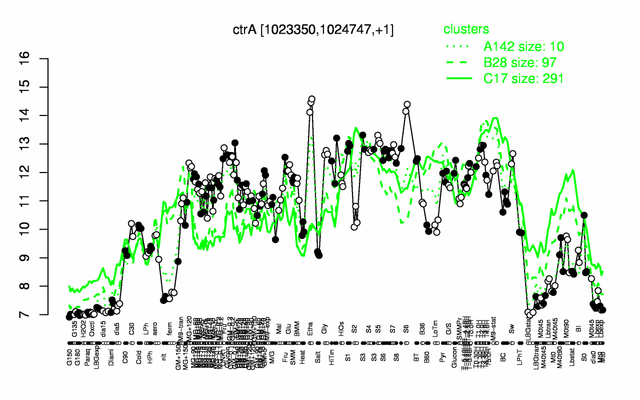
| |
Contents
Categories containing this gene/protein
transporters/ other, biosynthesis/ acquisition of amino acids, utilization of amino acids, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU09460
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: amino acid-polyamine-organocation (APC) superfamily (according to Swiss-Prot)
- Paralogous protein(s): YfnA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane PubMed
Database entries
- Structure:
- UniProt: O07576
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Boris R Belitsky, Abraham L Sonenshein
Roadblock repression of transcription by Bacillus subtilis CodY.
J Mol Biol: 2011, 411(4);729-43
[PubMed:21699902]
[WorldCat.org]
[DOI]
(I p)
Boris R Belitsky, Abraham L Sonenshein
Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis.
J Bacteriol: 2011, 193(2);473-84
[PubMed:21097623]
[WorldCat.org]
[DOI]
(I p)
Shaun R Brinsmade, Roelco J Kleijn, Uwe Sauer, Abraham L Sonenshein
Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools.
J Bacteriol: 2010, 192(24);6357-68
[PubMed:20935095]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Boris R Belitsky, Abraham L Sonenshein
Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis.
J Bacteriol: 2008, 190(4);1224-36
[PubMed:18083814]
[WorldCat.org]
[DOI]
(I p)
Virginie Molle, Yoshiko Nakaura, Robert P Shivers, Hirotake Yamaguchi, Richard Losick, Yasutaro Fujita, Abraham L Sonenshein
Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis.
J Bacteriol: 2003, 185(6);1911-22
[PubMed:12618455]
[WorldCat.org]
[DOI]
(P p)