Difference between revisions of "PerR"
| Line 1: | Line 1: | ||
| − | + | ||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || regulation of the response to peroxide | |style="background:#ABCDEF;" align="center"|'''Function''' || regulation of the response to peroxide | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU08730 perR] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/stress_response.html Stress]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/stress_response.html Stress]''' | ||
Revision as of 16:08, 6 August 2012
| Gene name | perR |
| Synonyms | ygaG |
| Essential | no |
| Product | transcriptional repressor (Fur family) |
| Function | regulation of the response to peroxide |
| Gene expression levels in SubtiExpress: perR | |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 16 kDa, 5.888 |
| Gene length, protein length | 435 bp, 145 aa |
| Immediate neighbours | ygaF, ygzB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 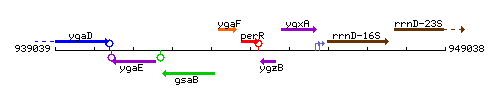
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The PerR regulon
The gene
Basic information
- Locus tag: BSU08730
Phenotypes of a mutant
- resistant to hydrogen peroxide, accumulates a porphyrin-like compound, and grows very slowly (due to heme sequestration by KatA) PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Fur family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- selective metal catalyzed oxidation of two histidine residues of the regulatory site results in induction (loss of DNA-binding activity) PubMed
- Cofactor(s): contains an Fe(2+) at the regulatory site and Zn(2+) PubMed
- Effectors of protein activity:
- responds to the presence of hydrogen peroxide
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P71086
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: perR PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant: HB0509 (spc), available in John Helmann's and Jörg Stülke's labs, also GP868 (fur::mls, perR::spc).
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Reviews
James M Dubbs, Skorn Mongkolsuk
Peroxide-sensing transcriptional regulators in bacteria.
J Bacteriol: 2012, 194(20);5495-503
[PubMed:22797754]
[WorldCat.org]
[DOI]
(I p)
Melinda J Faulkner, John D Helmann
Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis.
Antioxid Redox Signal: 2011, 15(1);175-89
[PubMed:20977351]
[WorldCat.org]
[DOI]
(I p)
Victor Duarte, Jean-Marc Latour
PerR vs OhrR: selective peroxide sensing in Bacillus subtilis.
Mol Biosyst: 2010, 6(2);316-23
[PubMed:20094649]
[WorldCat.org]
[DOI]
(I p)
Peter Zuber
Management of oxidative stress in Bacillus.
Annu Rev Microbiol: 2009, 63;575-97
[PubMed:19575568]
[WorldCat.org]
[DOI]
(I p)
David P Giedroc
Hydrogen peroxide sensing in Bacillus subtilis: it is all about the (metallo)regulator.
Mol Microbiol: 2009, 73(1);1-4
[PubMed:19508286]
[WorldCat.org]
[DOI]
(I p)
Original Publications