Difference between revisions of "FabD"
| Line 26: | Line 26: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:fabD_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:fabD_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=fabD_1663567_1664520_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:fabD_expression.png|500px]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | |||
<br/><br/><br/><br/><br/><br/> | <br/><br/><br/><br/><br/><br/> | ||
Revision as of 09:24, 19 April 2012
- Description: malonyl CoA-acyl carrier protein transacylase
| Gene name | fabD |
| Synonyms | ylpE |
| Essential | yes PubMed |
| Product | malonyl CoA-acyl carrier protein transacylase |
| Function | fatty acid biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 33 kDa, 4.515 |
| Gene length, protein length | 951 bp, 317 aa |
| Immediate neighbours | plsX, fabG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 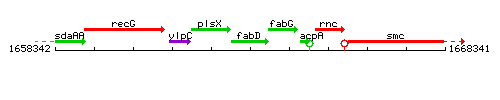
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed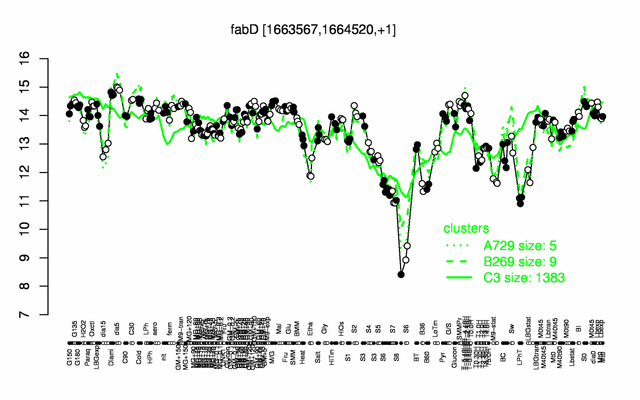
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15900
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Malonyl-CoA + [acyl-carrier-protein] = CoA + malonyl-[acyl-carrier-protein] (according to Swiss-Prot)
- Protein family: fabD family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P71019
- KEGG entry: [3]
- E.C. number: 2.3.1.39
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original Publications
Mariano A Martinez, Diego de Mendoza, Gustavo E Schujman
Transcriptional and functional characterization of the gene encoding acyl carrier protein in Bacillus subtilis.
Microbiology (Reading): 2010, 156(Pt 2);484-495
[PubMed:19850612]
[WorldCat.org]
[DOI]
(I p)
Gustavo E Schujman, Marcelo Guerin, Alejandro Buschiazzo, Francis Schaeffer, Leticia I Llarrull, Georgina Reh, Alejandro J Vila, Pedro M Alzari, Diego de Mendoza
Structural basis of lipid biosynthesis regulation in Gram-positive bacteria.
EMBO J: 2006, 25(17);4074-83
[PubMed:16932747]
[WorldCat.org]
[DOI]
(P p)
Natalia Comella, Alan D Grossman
Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis.
Mol Microbiol: 2005, 57(4);1159-74
[PubMed:16091051]
[WorldCat.org]
[DOI]
(P p)
Gustavo E Schujman, Luciana Paoletti, Alan D Grossman, Diego de Mendoza
FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis.
Dev Cell: 2003, 4(5);663-72
[PubMed:12737802]
[WorldCat.org]
[DOI]
(P p)
L Serre, E C Verbree, Z Dauter, A R Stuitje, Z S Derewenda
The Escherichia coli malonyl-CoA:acyl carrier protein transacylase at 1.5-A resolution. Crystal structure of a fatty acid synthase component.
J Biol Chem: 1995, 270(22);12961-4
[PubMed:7768883]
[WorldCat.org]
[DOI]
(P p)