Difference between revisions of "CheY"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 104: | Line 104: | ||
** ''[[ylxF]]-[[fliK]]-[[ylxG]]-[[flgE]]-[[fliL]]-[[fliM]]-[[fliY]]-[[cheY]]-[[fliZ]]-[[fliP]]-[[fliQ]]-[[fliR]]-[[flhB]]-[[flhA]]-[[flhF]]-[[ylxH]]-[[cheB]]-[[cheA]]-[[cheW]]-[[cheC]]-[[cheD]]-[[sigD]]-[[swrB]]'' {{PubMed|20233303}} | ** ''[[ylxF]]-[[fliK]]-[[ylxG]]-[[flgE]]-[[fliL]]-[[fliM]]-[[fliY]]-[[cheY]]-[[fliZ]]-[[fliP]]-[[fliQ]]-[[fliR]]-[[flhB]]-[[flhA]]-[[flhF]]-[[ylxH]]-[[cheB]]-[[cheA]]-[[cheW]]-[[cheC]]-[[cheD]]-[[sigD]]-[[swrB]]'' {{PubMed|20233303}} | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=cheY_1703834_1704196_1 cheY] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' | ||
** ''[[flgB]]'': [[SigA]] {{PubMed|9657996}}, [[SigD]] {{PubMed|9657996}} | ** ''[[flgB]]'': [[SigA]] {{PubMed|9657996}}, [[SigD]] {{PubMed|9657996}} | ||
** ''[[ylxF]]'': [[SigD]] {{PubMed|20233303}} | ** ''[[ylxF]]'': [[SigD]] {{PubMed|20233303}} | ||
Revision as of 09:03, 13 April 2012
- Description: two-component response regulator, modulation of flagellar switch bias
| Gene name | cheY |
| Synonyms | cheB |
| Essential | no |
| Product | two-component response regulator |
| Function | modulation of flagellar switch bias |
| Interactions involving this protein in SubtInteract: CheY | |
| MW, pI | 13 kDa, 4.746 |
| Gene length, protein length | 360 bp, 120 aa |
| Immediate neighbours | fliY, fliZ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 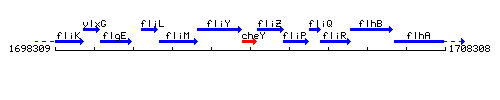
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
transcription factors and their control, motility and chemotaxis, phosphoproteins
This gene is a member of the following regulons
CodY regulon, SigD regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU16330
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated by CheA on an Asp residue
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P24072
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
- in minimal medium, CheY is present with 7,100 +/- 1,000 molecules per cell PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Gerald L Hazelbauer, Wing-Cheung Lai
Bacterial chemoreceptors: providing enhanced features to two-component signaling.
Curr Opin Microbiol: 2010, 13(2);124-32
[PubMed:20122866]
[WorldCat.org]
[DOI]
(I p)
Christopher V Rao, George W Ordal
The molecular basis of excitation and adaptation during chemotactic sensory transduction in bacteria.
Contrib Microbiol: 2009, 16;33-64
[PubMed:19494578]
[WorldCat.org]
[DOI]
(P p)
Christopher V Rao, John R Kirby, Adam P Arkin
Phosphatase localization in bacterial chemotaxis: divergent mechanisms, convergent principles.
Phys Biol: 2005, 2(3);148-58
[PubMed:16224120]
[WorldCat.org]
[DOI]
(I e)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Vincent J Cannistraro, George D Glekas, Christopher V Rao, George W Ordal
Cellular stoichiometry of the chemotaxis proteins in Bacillus subtilis.
J Bacteriol: 2011, 193(13);3220-7
[PubMed:21515776]
[WorldCat.org]
[DOI]
(I p)
Y Pazy, M A Motaleb, M T Guarnieri, N W Charon, R Zhao, R E Silversmith
Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate.
Proc Natl Acad Sci U S A: 2010, 107(5);1924-9
[PubMed:20080618]
[WorldCat.org]
[DOI]
(I p)
Travis J Muff, George W Ordal
The CheC phosphatase regulates chemotactic adaptation through CheD.
J Biol Chem: 2007, 282(47);34120-8
[PubMed:17908686]
[WorldCat.org]
[DOI]
(P p)
Kazuo Kobayashi
Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis.
Mol Microbiol: 2007, 66(2);395-409
[PubMed:17850253]
[WorldCat.org]
[DOI]
(P p)
Travis J Muff, Richard M Foster, Peter J Y Liu, George W Ordal
CheX in the three-phosphatase system of bacterial chemotaxis.
J Bacteriol: 2007, 189(19);7007-13
[PubMed:17675386]
[WorldCat.org]
[DOI]
(P p)
H Werhane, P Lopez, M Mendel, M Zimmer, G W Ordal, L M Márquez-Magaña
The last gene of the fla/che operon in Bacillus subtilis, ylxL, is required for maximal sigmaD function.
J Bacteriol: 2004, 186(12);4025-9
[PubMed:15175317]
[WorldCat.org]
[DOI]
(P p)
Hendrik Szurmant, Travis J Muff, George W Ordal
Bacillus subtilis CheC and FliY are members of a novel class of CheY-P-hydrolyzing proteins in the chemotactic signal transduction cascade.
J Biol Chem: 2004, 279(21);21787-92
[PubMed:14749334]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
Hendrik Szurmant, Michael W Bunn, Vincent J Cannistraro, George W Ordal
Bacillus subtilis hydrolyzes CheY-P at the location of its action, the flagellar switch.
J Biol Chem: 2003, 278(49);48611-6
[PubMed:12920116]
[WorldCat.org]
[DOI]
(P p)
J R Kirby, M M Saulmon, C J Kristich, G W Ordal
CheY-dependent methylation of the asparagine receptor, McpB, during chemotaxis in Bacillus subtilis.
J Biol Chem: 1999, 274(16);11092-100
[PubMed:10196193]
[WorldCat.org]
[DOI]
(P p)
W Estacio, S S Anna-Arriola, M Adedipe, L M Márquez-Magaña
Dual promoters are responsible for transcription initiation of the fla/che operon in Bacillus subtilis.
J Bacteriol: 1998, 180(14);3548-55
[PubMed:9657996]
[WorldCat.org]
[DOI]
(P p)
J R Kirby, C J Kristich, S L Feinberg, G W Ordal
Methanol production during chemotaxis to amino acids in Bacillus subtilis.
Mol Microbiol: 1997, 24(4);869-78
[PubMed:9194713]
[WorldCat.org]
[DOI]
(P p)
L M Márquez-Magaña, M J Chamberlin
Characterization of the sigD transcription unit of Bacillus subtilis.
J Bacteriol: 1994, 176(8);2427-34
[PubMed:8157612]
[WorldCat.org]
[DOI]
(P p)
D S Bischoff, R B Bourret, M L Kirsch, G W Ordal
Purification and characterization of Bacillus subtilis CheY.
Biochemistry: 1993, 32(35);9256-61
[PubMed:8369293]
[WorldCat.org]
[DOI]
(P p)