Difference between revisions of "FeuA"
(→Database entries) |
(→References) |
||
| Line 144: | Line 144: | ||
=References= | =References= | ||
| − | + | <pubmed>21802011,20086155,19746494,19673474,14563870,16672620,10092453,16889643,17725565,12354229,18957862,18763711</pubmed> | |
| − | <pubmed>20086155,19746494,19673474,14563870,16672620,10092453,16889643,17725565,12354229,18957862 18763711</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:39, 2 August 2011
- Description: ABC transporter for the siderophores Fe-enterobactin and Fe-bacillibactin (binding protein), with YusV as ATPase
| Gene name | feuA |
| Synonyms | |
| Essential | no |
| Product | ABC transporter for the siderophores Fe-enterobactin and Fe-bacillibactin (binding protein) |
| Function | acquisition of iron |
| Interactions involving this protein in SubtInteract: FeuA | |
| Metabolic function and regulation of this protein in SubtiPathways: Stress, Metal ion homeostasis | |
| MW, pI | 34 kDa, 8.018 |
| Gene length, protein length | 951 bp, 317 aa |
| Immediate neighbours | feuB, btr |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 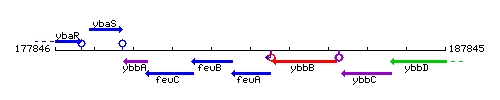
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
ABC transporters, acquisition of iron, iron metabolism, membrane proteins
This gene is a member of the following regulons
Btr regulon, CitB regulon, Fur regulon
The gene
Basic information
- Locus tag: BSU01630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/biological activity:
Substrate binding protein for the triscatecholate siderophores ferri-bacillibactin and ferri-enterobactin and part of the FeuA-FeuB-FeuC-YusV ATP-binding cassette-type transporter. Furthermore ferric complexes of L-norepinephrine are bound by this protein.
- Protein family: Fe/B12 periplasmic-binding domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
Two independent non-symmetric globular domains, connected by a long alpha-helix (residues 143-164 of the mature protein).
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 2PHZ, 2WI8, 2WHY (complex with ferri-bacillibactin), 2XUZ (complex with ferri-enterobactin), 2XV1 (complex with ferric mecam)
- UniProt: P40409
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References