Difference between revisions of "AcpS"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | |style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/AcpS AcpS] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | ||
Revision as of 14:36, 29 July 2011
- Description: acyl-carrier protein synthase, 4'-phosphopantetheine transferase
| Gene name | acpS |
| Synonyms | ydcB |
| Essential | yes PubMed |
| Product | acyl-carrier protein synthase |
| Function | fatty acid biosynthesis |
| Interactions involving this protein in SubtInteract: AcpS | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 13 kDa, 9.915 |
| Gene length, protein length | 363 bp, 121 aa |
| Immediate neighbours | ydcA, ydcC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 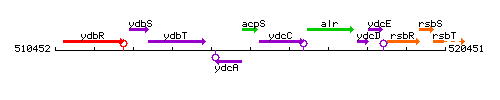
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU04620
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: CoA-(4'-phosphopantetheine) + apo-[acyl-carrier-protein] = adenosine 3',5'-bisphosphate + holo-[acyl-carrier-protein] (according to Swiss-Prot)
- Protein family: AcpS family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): magnesium PubMed
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P96618
- KEGG entry: [2]
- E.C. number: 2.7.8.7
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original Publications
Juergen J May, Robert Finking, Frank Wiegeshoff, Thomas T Weber, Nina Bandur, Ulrich Koert, Mohamed A Marahiel
Inhibition of the D-alanine:D-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium's susceptibility to antibiotics that target the cell wall.
FEBS J: 2005, 272(12);2993-3003
[PubMed:15955059]
[WorldCat.org]
[DOI]
(P p)
Mohammad Reza Mofid, Robert Finking, Mohamed A Marahiel
Recognition of hybrid peptidyl carrier proteins/acyl carrier proteins in nonribosomal peptide synthetase modules by the 4'-phosphopantetheinyl transferases AcpS and Sfp.
J Biol Chem: 2002, 277(19);17023-31
[PubMed:11867633]
[WorldCat.org]
[DOI]
(P p)
H D Mootz, R Finking, M A Marahiel
4'-phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis.
J Biol Chem: 2001, 276(40);37289-98
[PubMed:11489886]
[WorldCat.org]
[DOI]
(P p)
K D Parris, L Lin, A Tam, R Mathew, J Hixon, M Stahl, C C Fritz, J Seehra, W S Somers
Crystal structures of substrate binding to Bacillus subtilis holo-(acyl carrier protein) synthase reveal a novel trimeric arrangement of molecules resulting in three active sites.
Structure: 2000, 8(8);883-95
[PubMed:10997907]
[WorldCat.org]
[DOI]
(P p)