Difference between revisions of "DnaB"
(→References) |
(→References) |
||
| Line 120: | Line 120: | ||
=References= | =References= | ||
| − | <pubmed> 19707554 15686560,12718886,2554322,11250911,15186423,11842108,11585815, 16479537, 19415803, 14757052, 10844689, 12974630 19968790 20071750 </pubmed> | + | <pubmed> 19707554 15686560,12718886,2554322,11250911,15186423,11842108,11585815, 16479537, 19415803, 14757052, 10844689, 12974630 19968790 20071750 11679082 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:23, 15 January 2010
- Description: initiation of chromosome replication/ membrane attachment protein
| Gene name | dnaB |
| Synonyms | |
| Essential | yes PubMed |
| Product | initiation of chromosome replication/ membrane attachment protein |
| Function | DNA replication |
| MW, pI | 54 kDa, 5.278 |
| Gene length, protein length | 1416 bp, 472 aa |
| Immediate neighbours | dnaI, ytcG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 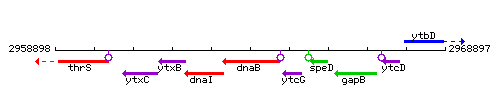
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28990
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P07908
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References