Difference between revisions of "CspB"
| Line 122: | Line 122: | ||
<pubmed>8321288,8321289,9533624,9379903,8294017,8755892,9914312,11591689,7476164,1409560,16352840,9920884,1400185,12427936,8307174,7703860,16352840, </pubmed> | <pubmed>8321288,8321289,9533624,9379903,8294017,8755892,9914312,11591689,7476164,1409560,16352840,9920884,1400185,12427936,8307174,7703860,16352840, </pubmed> | ||
| − | |||
| − | |||
| − | |||
Revision as of 13:08, 15 June 2009
- Description: major cold-shock protein
| Gene name | cspB |
| Synonyms | |
| Essential | no |
| Product | major cold-shock protein |
| Function | RNA chaperone |
| MW, pI | 7 kDa, 4.341 |
| Gene length, protein length | 201 bp, 67 aa |
| Immediate neighbours | yhcI, yhcJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 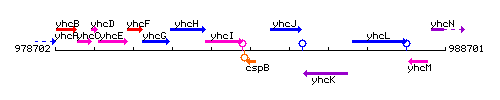
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU09100
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot), cytoplasma, colocalizes with the ribosomes PubMed
Database entries
- Swiss prot entry: P32081
- KEGG entry: [3]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Mohamed Marahiel, Marburg University, Germany homepage
Your additional remarks
References