Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' Transcriptional activator of the ''gltAB'' operon. Activates expression of the operon in the absence of arginine. <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''gltC'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || No |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || transcriptional regulator (LysR family) |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || positive regulation <br/>of the glutamate synthase operon (''gltAB'') |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 33.9 kDa, 5.62 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 900 bp, 300 amino acids |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[gltA]]'', ''[[proJ]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB13729]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |- |
| + | |- | ||
| + | |colspan="2" | '''Genetic context''' <br/> [[Image:gltC_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 29: | Line 31: | ||
__TOC__ | __TOC__ | ||
| − | <br/><br/> | + | <br/><br/><br/><br/><br/> |
=The gene= | =The gene= | ||
| Line 35: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU18460 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | ''gltC'' mutants are auxotrophic for glutamate. | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/gltC.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:'''[http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10810] |
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 52: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' transcription activation of the ''[[gltA]]-[[gltB]]'' operon [http://www.ncbi.nlm.nih.gov/sites/entrez/2548995 PubMed] |
| − | * '''Protein family:''' | + | * '''Protein family:''' glutamate synthase family (according to Swiss-Prot) LysR-type transcription regulator [http://www.ncbi.nlm.nih.gov/sites/entrez/2548995 PubMed] |
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' none, but there are 19 members of the LysR family in ''B. subtilis'' |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 62: | Line 64: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''Domains:''' DNA-binding helix-turn-helix motif: AA 18 ... 37 |
* '''Modification:''' | * '''Modification:''' | ||
| Line 68: | Line 70: | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:''' 2-oxoglutarate stimulates transcription activation, glutamate inhibits transcription activation [http://www.ncbi.nlm.nih.gov/sites/entrez/17134717 PubMed] |
* '''Interactions:''' | * '''Interactions:''' | ||
| + | ** GltC-[[RocG]], This interaction takes place in the presence of glutamate. It prevents the transcription activation of the ''[[gltA]]-[[gltB]]'' operon. Note that [[RocG]] expression is strongly regulated by carbon and nitrogen sources, respectively. [http://www.ncbi.nlm.nih.gov/sites/entrez/17608797 PubMed] | ||
| − | * '''Localization:''' | + | * '''Localization:''' |
=== Database entries === | === Database entries === | ||
| Line 78: | Line 81: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P20668 P20668] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU18460] |
| − | |||
| − | |||
=== Additional information=== | === Additional information=== | ||
| Line 88: | Line 89: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[gltC]]'' |
| − | * ''' | + | * '''Sigma factor:''' [[SigA]] |
| − | * '''Regulation:''' | + | * '''Regulation:''' autoregulation by GltC |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' autorepression |
| − | * '''Additional information:''' | + | * '''Database entries:''' [http://dbtbs.hgc.jp/COG/prom/gltC.html DBTBS] |
| + | |||
| + | * ''' Additional information:''' | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' GP344 (erm), GP738 (spc) (available in [[Stülke]] lab) |
| − | * '''Expression vector:''' | + | * '''Expression vector:''' pGP903 (in [[pWH844]], N-terminal His-tag), pGP951 (C-terminal Strep-tag) (available in [[Stülke]] lab) |
| − | + | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' ''B. pertussis'' adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Stülke]] lab |
| − | * '''Antibody:''' | + | * '''Antibody:''' available in Stülke lab |
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| + | |||
| + | [[Linc Sonenshein|Linc Sonenshein]], Tufts University, Boston, MA, USA [http://www.tufts.edu/sackler/microbiology/faculty/sonenshein/index.html Homepage] | ||
| + | |||
| + | [[Stülke|Jörg Stülke]], University of Göttingen, Germany | ||
| + | [http://wwwuser.gwdg.de/~genmibio/stuelke.html Homepage] | ||
=Your additional remarks= | =Your additional remarks= | ||
| Line 118: | Line 126: | ||
=References= | =References= | ||
| − | <pubmed> | + | <pubmed>7559360 15150225 2548995 17183217 17608797 17134717 14523131, </pubmed> |
| − | # | + | # Yoshida K, et al. (2003) Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. ''Mol Microbiol'' '''49(1):''' 157-65. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+12823818 PubMed] |
| − | # | + | # Belitsky, B. R., and Sonenshein, A. L. (1995) Mutations in GltC that increase ''Bacillus subtilis gltA'' expression. J Bacteriol 177: 5696-5700.[http://www.ncbi.nlm.nih.gov/sites/entrez/7559360 PubMed] |
| + | # Belitsky, B. R., and Sonenshein, A. L. (2004) Modulation of activity of ''Bacillus subtilis'' regulatory proteins GltC and TnrA by glutamate dehydrogenase. J Bacteriol 186: 3399-3407.[http://www.ncbi.nlm.nih.gov/sites/entrez/15150225 PubMed] | ||
| + | # Bohannon, D. E., and Sonenshein, A. L. (1989) Positive regulation of glutamate biosynthesis in ''Bacillus subtilis''. J Bacteriol 171: 4718-4727.[http://www.ncbi.nlm.nih.gov/sites/entrez/2548995 PubMed] | ||
| + | # Commichau, F. M., Wacker, I., Schleider, J., Blencke, H.-M., Reif, I., Tripal, P., and Stülke, J. (2007) Characterization of ''Bacillus subtilis'' mutants with carbon source-independent glutamate biosynthesis. J Mol Microbiol Biotechnol 12: 106-113. [http://www.ncbi.nlm.nih.gov/sites/entrez/17183217 PubMed] | ||
| + | # Commichau, F. M., Herzberg, C., Tripal, P., Valerius, O., and Stülke, J. (2007) A regulatory protein-protein interaction governs glutamate biosynthesis in ''Bacillus subtilis'': The glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol Microbiol 65: 642-654. [http://www.ncbi.nlm.nih.gov/sites/entrez/17608797 PubMed] | ||
| + | # Picossi, S., Belitsky, B. R., and Sonenshein, A. L. (2007) Molecular mechanism of the regulation of ''Bacillus subtilis gltAB'' expression by GltC. J Mol Biol 365: 1298-1313. [http://www.ncbi.nlm.nih.gov/sites/entrez/17134717 PubMed] | ||
| + | # Wacker, I., Ludwig, H., Reif, I., Blencke, H. M., Detsch, C., and Stülke, J. (2003) The regulatory link between carbon and nitrogen metabolism in ''Bacillus subtilis'': regulation of the ''gltAB'' operon by the catabolite control protein CcpA. Microbiology 149: 3001-3009.[http://www.ncbi.nlm.nih.gov/sites/entrez/14523131 PubMed] | ||
| + | # Herzberg, C., Flórez Weidinger, L. A., Dörrbecker, B., Hübner, S., Stülke, J. & Commichau, F. M. (2007) SPINE: A method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7: 4032-4035. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17994626 PubMed] | ||
| + | # Schell, M. A. (1993). Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol 47, 597-626. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+8257110 PubMed] | ||
Revision as of 13:19, 8 June 2009
- Description: Transcriptional activator of the gltAB operon. Activates expression of the operon in the absence of arginine.
| Gene name | gltC |
| Synonyms | |
| Essential | No |
| Product | transcriptional regulator (LysR family) |
| Function | positive regulation of the glutamate synthase operon (gltAB) |
| MW, pI | 33.9 kDa, 5.62 |
| Gene length, protein length | 900 bp, 300 amino acids |
| Immediate neighbours | gltA, proJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 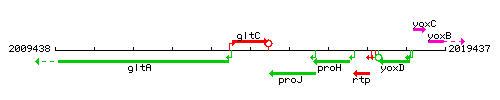
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU18460
Phenotypes of a mutant
gltC mutants are auxotrophic for glutamate.
Database entries
- DBTBS entry: [1]
- SubtiList entry:[2]
Additional information
The protein
Basic information/ Evolution
- Protein family: glutamate synthase family (according to Swiss-Prot) LysR-type transcription regulator PubMed
- Paralogous protein(s): none, but there are 19 members of the LysR family in B. subtilis
Extended information on the protein
- Kinetic information:
- Domains: DNA-binding helix-turn-helix motif: AA 18 ... 37
- Modification:
- Cofactor(s):
- Effectors of protein activity: 2-oxoglutarate stimulates transcription activation, glutamate inhibits transcription activation PubMed
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P20668
- KEGG entry: [3]
Additional information
Expression and regulation
- Operon: gltC
- Sigma factor: SigA
- Regulation: autoregulation by GltC
- Regulatory mechanism: autorepression
- Database entries: DBTBS
- Additional information:
Biological materials
- Mutant: GP344 (erm), GP738 (spc) (available in Stülke lab)
- Expression vector: pGP903 (in pWH844, N-terminal His-tag), pGP951 (C-terminal Strep-tag) (available in Stülke lab)
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody: available in Stülke lab
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Yoshida K, et al. (2003) Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol Microbiol 49(1): 157-65. PubMed
- Belitsky, B. R., and Sonenshein, A. L. (1995) Mutations in GltC that increase Bacillus subtilis gltA expression. J Bacteriol 177: 5696-5700.PubMed
- Belitsky, B. R., and Sonenshein, A. L. (2004) Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase. J Bacteriol 186: 3399-3407.PubMed
- Bohannon, D. E., and Sonenshein, A. L. (1989) Positive regulation of glutamate biosynthesis in Bacillus subtilis. J Bacteriol 171: 4718-4727.PubMed
- Commichau, F. M., Wacker, I., Schleider, J., Blencke, H.-M., Reif, I., Tripal, P., and Stülke, J. (2007) Characterization of Bacillus subtilis mutants with carbon source-independent glutamate biosynthesis. J Mol Microbiol Biotechnol 12: 106-113. PubMed
- Commichau, F. M., Herzberg, C., Tripal, P., Valerius, O., and Stülke, J. (2007) A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: The glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol Microbiol 65: 642-654. PubMed
- Picossi, S., Belitsky, B. R., and Sonenshein, A. L. (2007) Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC. J Mol Biol 365: 1298-1313. PubMed
- Wacker, I., Ludwig, H., Reif, I., Blencke, H. M., Detsch, C., and Stülke, J. (2003) The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149: 3001-3009.PubMed
- Herzberg, C., Flórez Weidinger, L. A., Dörrbecker, B., Hübner, S., Stülke, J. & Commichau, F. M. (2007) SPINE: A method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7: 4032-4035. PubMed
- Schell, M. A. (1993). Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol 47, 597-626. PubMed