Difference between revisions of "SecA"
(→Biological materials) |
(→Extended information on the protein) |
||
| Line 64: | Line 64: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''Domains:''' nucleotide binding domain, preprotein binding domain, IRA2 domain, scaffold domain, wing domain, IRA1 domain, C-terminal domain |
* '''Modification:''' | * '''Modification:''' | ||
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):'''Magnesium |
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:''' anionic phospholipids, preprotein, SecY |
| − | * '''Interactions:''' [[SecA]]-[[FfH]], [[CsaA]]-[[SecA]], [[SecA]]-[[SecY]] | + | * '''Interactions:''' [[SecA]]-[[FfH]], [[CsaA]]-[[SecA]], [[SecA]]-[[SecY]], [[SecA]]-[[SecA]] |
* '''Localization:''' cell membrane (according to Swiss-Prot) | * '''Localization:''' cell membrane (according to Swiss-Prot) | ||
Revision as of 11:40, 2 June 2009
- Description: preprotein translocase subunit (ATPase)
| Gene name | secA |
| Synonyms | div, div-341, ts-341 |
| Essential | yes PubMed |
| Product | preprotein translocase subunit (ATPase) |
| Function | protein secretion |
| MW, pI | 95 kDa, 5.34 |
| Gene length, protein length | 2523 bp, 841 aa |
| Immediate neighbours | prfB, yvyD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 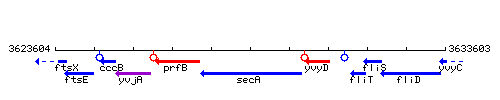
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: secA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: nucleotide binding domain, preprotein binding domain, IRA2 domain, scaffold domain, wing domain, IRA1 domain, C-terminal domain
- Modification:
- Cofactor(s):Magnesium
- Effectors of protein activity: anionic phospholipids, preprotein, SecY
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Swiss prot entry: P28366
- KEGG entry: BSU35300
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: