Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' initiation of chromosome replication/ membrane attachment protein <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''dnaB'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || initiation of chromosome replication/ membrane attachment protein |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || DNA replication |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 54 kDa, 5.278 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1416 bp, 472 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[dnaI]]'', ''[[ytcG]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Hier soll was neues rein''' | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:dnaB_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 35: | Line 35: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Coordinates:''' | + | * '''Coordinates:''' |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | |||
| + | essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' | + | * '''DBTBS entry:''' no entry |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10358] |
=== Additional information=== | === Additional information=== | ||
| + | |||
=The protein= | =The protein= | ||
| Line 51: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' |
| − | * '''Protein family:''' | + | * '''Protein family:''' |
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 62: | Line 65: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | |||
| − | |||
* '''Modification:''' | * '''Modification:''' | ||
| Line 71: | Line 72: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''Interactions:''' [[DnaX]]-[[DnaB]] [http://www.ncbi.nlm.nih.gov/sites/entrez/14757052 PubMed] |
| − | * '''Localization:''' | + | * '''Localization:''' Membrane-proximal (Spotty) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] forms foci, close to oriC [http://www.ncbi.nlm.nih.gov/sites/entrez/10844689 PubMed] |
=== Database entries === | === Database entries === | ||
| Line 79: | Line 80: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''Swiss prot entry:''' | + | * '''Swiss prot entry:''' |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU28990] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' |
=== Additional information=== | === Additional information=== | ||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:' | + | * '''Operon:''' |
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
| − | * '''Regulation:''' | + | * '''Regulation:''' |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' |
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| Line 105: | Line 105: | ||
* '''Expression vector:''' | * '''Expression vector:''' | ||
| − | + | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| + | |||
| + | * '''two-hybrid system:''' | ||
* '''Antibody:''' | * '''Antibody:''' | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | |||
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 120: | Line 120: | ||
=References= | =References= | ||
| − | |||
# Meile et al. (2006) Systematic localisation of proteins fused to the green fluorescent protein in ''Bacillus subtilis'': identification of new proteins at the DNA replication factory ''Proteomics'' '''6:''' 2135-2146. [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] | # Meile et al. (2006) Systematic localisation of proteins fused to the green fluorescent protein in ''Bacillus subtilis'': identification of new proteins at the DNA replication factory ''Proteomics'' '''6:''' 2135-2146. [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] | ||
| − | # | + | # Haroniti et al. (2004) The clamp-loader-helicase interaction in ''Bacillus''. Atomic force microscopy reveals the structural organisation of the DnaB-tau complex in ''Bacillus''.''J. Mol. Biol.'' '''336:''' 381-393. [http://www.ncbi.nlm.nih.gov/sites/entrez/14757052 PubMed] |
| − | # | + | # Imai et al. (2000) Subcellular localization of Dna-initiation proteins of ''Bacillus subtilis'': evidence that chromosome replication begins at either edge of the nucleoids. ''Mol. Microbiol.'' '''36:''' 1037-1048. [http://www.ncbi.nlm.nih.gov/sites/entrez/10844689 PubMed] |
| − | |||
Revision as of 17:20, 15 April 2009
- Description: initiation of chromosome replication/ membrane attachment protein
| Gene name | dnaB |
| Synonyms | |
| Essential | yes PubMed |
| Product | initiation of chromosome replication/ membrane attachment protein |
| Function | DNA replication |
| MW, pI | 54 kDa, 5.278 |
| Gene length, protein length | 1416 bp, 472 aa |
| Immediate neighbours | dnaI, ytcG |
| Hier soll was neues rein | |
Genetic context 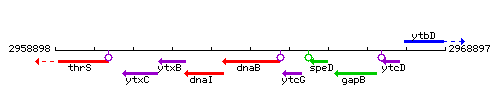
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Meile et al. (2006) Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory Proteomics 6: 2135-2146. PubMed
- Haroniti et al. (2004) The clamp-loader-helicase interaction in Bacillus. Atomic force microscopy reveals the structural organisation of the DnaB-tau complex in Bacillus.J. Mol. Biol. 336: 381-393. PubMed
- Imai et al. (2000) Subcellular localization of Dna-initiation proteins of Bacillus subtilis: evidence that chromosome replication begins at either edge of the nucleoids. Mol. Microbiol. 36: 1037-1048. PubMed