Difference between revisions of "PdhD"
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || dihydrolipoamide dehydrogenase E3 subunit of | + | |style="background:#ABCDEF;" align="center"| '''Product''' || dihydrolipoamide dehydrogenase E3 subunit<br/> of both pyruvate dehydrogenase and 2-oxoglutarate<br/> dehydrogenase complexes |
| − | |||
| − | both pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || links glycolysis and TCA cycle, enzyme in TCA cycle | |style="background:#ABCDEF;" align="center"|'''Function''' || links glycolysis and TCA cycle, enzyme in TCA cycle | ||
| Line 32: | Line 30: | ||
__TOC__ | __TOC__ | ||
| − | <br/><br/> | + | <br/><br/><br/><br/> |
=The gene= | =The gene= | ||
Revision as of 10:24, 31 March 2009
- Description: dihydrolipoamide dehydrogenase E3 subunit of both pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes
| Gene name | pdhD |
| Synonyms | citL |
| Essential | no |
| Product | dihydrolipoamide dehydrogenase E3 subunit of both pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes |
| Function | links glycolysis and TCA cycle, enzyme in TCA cycle |
| MW, pI | 49 kDa, 4.76 |
| Gene length, protein length | 1410 bp, 470 aa |
| Immediate neighbours | pdhC, slp |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 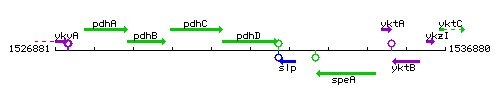
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
defects in sporulation and unable to grow on glucose as single carbon source PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 1EBD (complex with binding domain of dihydrolipoamide acetylase, Geobacillus stearothermophilus)
- Swiss prot entry:
- KEGG entry: [3]
- E.C. number: 1.8.1.4
Additional information
Expression and regulation
- Sigma factor: SigA
- Regulation: weak induction by glucose PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: