Difference between revisions of "FtsZ"
(→Database entries) |
(→Other original Publications) |
||
| Line 182: | Line 182: | ||
==Other original Publications== | ==Other original Publications== | ||
| − | <pubmed> 24007276 15288790, 15317782,12180929, 9364910,10323866, 19212404,15942012, 12007411,16420366, 25176632 16159787,10747015, 16950129, 16796675,10322023, 9495766,9287012,1569582,10878122, 17718511 11395470, 10449747, 17662947, 12368265,18284588,8600030,18588879,7592498, 19136590 , 19429628, 19141479 19843223 16484179 20199598 20566861 20711458 20807205 20933427 15948963 12700262 22298780 22457634 22730127 22984350 23577149 22931116,22912848,21224850 23692518 23701187 23836667 16159787 24300445 24316672 24825009 18573169 25403286,25358088 </pubmed> | + | <pubmed> 24007276 15288790, 15317782,12180929, 9364910,10323866, 19212404,15942012, 12007411,16420366, 25176632 16159787,10747015, 16950129, 16796675,10322023, 9495766,9287012,1569582,10878122, 17718511 11395470, 10449747, 17662947, 12368265,18284588,8600030,18588879,7592498, 19136590 , 19429628, 19141479 19843223 16484179 20199598 20566861 20711458 20807205 20933427 15948963 12700262 22298780 22457634 22730127 22984350 23577149 22931116,22912848,21224850 23692518 23701187 23836667 16159787 24300445 24316672 24825009 18573169 25403286,25358088 25848052</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 07:03, 9 April 2015
- Description: cell-division initiation protein (septum formation)
| Gene name | ftsZ |
| Synonyms | ts-1 |
| Essential | yes PubMed |
| Product | cell-division initiation protein (septum formation) |
| Function | formation of Z-ring |
| Gene expression levels in SubtiExpress: ftsZ | |
| Interactions involving this protein in SubtInteract: FtsZ | |
| MW, pI | 40 kDa, 4.814 |
| Gene length, protein length | 1146 bp, 382 aa |
| Immediate neighbours | ftsA, bpr |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 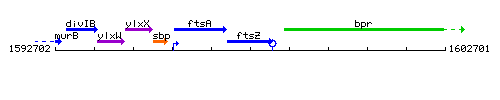
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed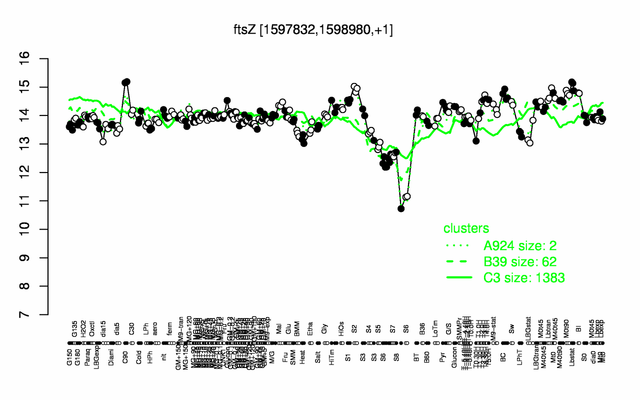
| |
Contents
Categories containing this gene/protein
cell division, essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15290
Phenotypes of a mutant
Database entries
- BsubCyc: BSU15290
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsZ family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Z ring formation is inhibited upon binding of MciZ to FtsZ
- bundling of FtsZ protofilaments into strikingly long and regular tubular structures reminiscent of eukaryotic microtubules requires the prior formation of large ring polymers of SepF PubMed
- interaction with UgtP inhibits FtsZ filament formation PubMed
- FtsZ polymerization is inhibited by interaction with MinC PubMed
- Z ring formation requires PdhA in a pyruvate-dependent manner PubMed
- Localization:
- septal at the cell membrane PubMed
- septal localization partially depends on the proton motive force PubMed
- Noc and the Min system ensure the efficient utilization of the division site at midcell in by ensuring Z ring placement PubMed
- FtsZ is anchored to the cell membrane by either FtsA or SepF PubMed
Database entries
- BsubCyc: BSU15290
- UniProt: P17865
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- strains:
- GP1372 (Pxyl ftsZ aphA3) disA::tet cdaS::ermC for xylose inducible expression of ftsZ, available in Jörg Stülke's lab
- Expression vector:
- GP2009: expression of ftsZ-Strep under control of the ftsZ promoter (based on pGP1389), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody: available in the Jeff Errington lab
Labs working on this gene/protein
- Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
- Leendert Hamoen, CBCB, Newcastle University, UK
Your additional remarks
References
Reviews
Leigh G Monahan, Andrew T F Liew, Amy L Bottomley, Elizabeth J Harry
Division site positioning in bacteria: one size does not fit all.
Front Microbiol: 2014, 5;19
[PubMed:24550892]
[WorldCat.org]
[DOI]
(P e)
An-Chun Chien, Norbert S Hill, Petra Anne Levin
Cell size control in bacteria.
Curr Biol: 2012, 22(9);R340-9
[PubMed:22575476]
[WorldCat.org]
[DOI]
(I p)
Christine Kaimer, Peter L Graumann
Players between the worlds: multifunctional DNA translocases.
Curr Opin Microbiol: 2011, 14(6);719-25
[PubMed:22047950]
[WorldCat.org]
[DOI]
(I p)
Clare L Kirkpatrick, Patrick H Viollier
New(s) to the (Z-)ring.
Curr Opin Microbiol: 2011, 14(6);691-7
[PubMed:21981908]
[WorldCat.org]
[DOI]
(I p)
Harold P Erickson, David E Anderson, Masaki Osawa
FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one.
Microbiol Mol Biol Rev: 2010, 74(4);504-28
[PubMed:21119015]
[WorldCat.org]
[DOI]
(I p)
Matthew T Cabeen, Christine Jacobs-Wagner
The bacterial cytoskeleton.
Annu Rev Genet: 2010, 44;365-92
[PubMed:21047262]
[WorldCat.org]
[DOI]
(I p)
Marc Bramkamp, Suey van Baarle
Division site selection in rod-shaped bacteria.
Curr Opin Microbiol: 2009, 12(6);683-8
[PubMed:19884039]
[WorldCat.org]
[DOI]
(I p)
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Peter L Graumann
Cytoskeletal elements in bacteria.
Annu Rev Microbiol: 2007, 61;589-618
[PubMed:17506674]
[WorldCat.org]
[DOI]
(P p)
Linda A Amos, Fusinita van den Ent, Jan Löwe
Structural/functional homology between the bacterial and eukaryotic cytoskeletons.
Curr Opin Cell Biol: 2004, 16(1);24-31
[PubMed:15037301]
[WorldCat.org]
[DOI]
(P p)
FtsZ as antibacterial drug target
Other original Publications