Difference between revisions of "Cah"
| Line 120: | Line 120: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
** number of protein molecules per cell (complex medium with amino acids, without glucose): 205 {{PubMed|24696501}} | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 205 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 980 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1680 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 13:33, 17 April 2014
- Description: cephalosporin C deacetylase
| Gene name | cah |
| Synonyms | |
| Essential | no |
| Product | cephalosporin C deacetylase) |
| Function | resistance to cephalosporin C |
| Gene expression levels in SubtiExpress: cah | |
| MW, pI | 35 kDa, 5.419 |
| Gene length, protein length | 954 bp, 318 aa |
| Immediate neighbours | ycgK, ycgL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 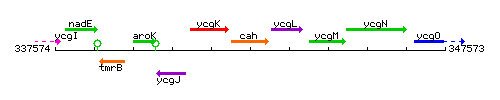
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed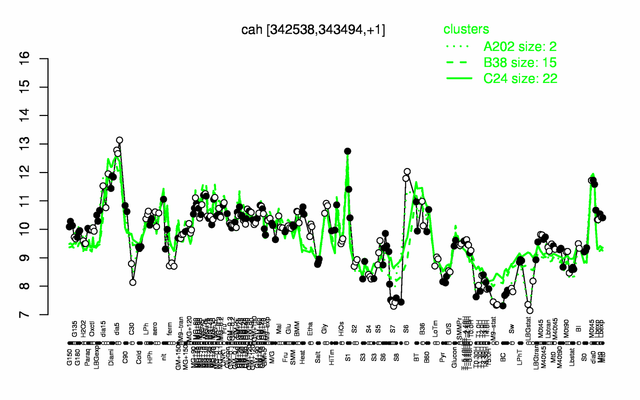
| |
Contents
Categories containing this gene/protein
resistance against toxins/ antibiotics
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU03180
Phenotypes of a mutant
Database entries
- BsubCyc: BSU03180
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Deacetylation of xylans and xylo-oligosaccharides (according to Swiss-Prot)
- Protein family: carbohydrate esterase 7 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU03180
- Structure: 1ODS
- UniProt: P94388
- KEGG entry: [3]
- E.C. number: 3.1.1.41
Additional information
Expression and regulation
- Operon: cah PubMed
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1680 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
A Takimoto, S Yagi, K Mitsushima
High-level expression, purification, and some properties of a recombinant cephalosporin-C deacetylase.
J Biosci Bioeng: 1999, 87(4);456-62
[PubMed:16232499]
[WorldCat.org]
[DOI]
(P p)
Akio Takimoto, Tomoaki Takakura, Hiroyoshi Tani, Shigeo Yagi, Kenji Mitsushima
Batch production of deacetyl 7-aminocephalosporanic acid by immobilized cephalosporin-C deacetylase.
Appl Microbiol Biotechnol: 2004, 65(3);263-7
[PubMed:15069587]
[WorldCat.org]
[DOI]
(P p)
Florence Vincent, Simon J Charnock, Koen H G Verschueren, Johan P Turkenburg, David J Scott, Wendy A Offen, Shirley Roberts, Gavin Pell, Harry J Gilbert, Gideon J Davies, James A Brannigan
Multifunctional xylooligosaccharide/cephalosporin C deacetylase revealed by the hexameric structure of the Bacillus subtilis enzyme at 1.9A resolution.
J Mol Biol: 2003, 330(3);593-606
[PubMed:12842474]
[WorldCat.org]
[DOI]
(P p)
K Mitsushima, A Takimoto, T Sonoyama, S Yagi
Gene cloning, nucleotide sequence, and expression of a cephalosporin-C deacetylase from Bacillus subtilis.
Appl Environ Microbiol: 1995, 61(6);2224-9
[PubMed:7793942]
[WorldCat.org]
[DOI]
(P p)