Difference between revisions of "MnaA"
| Line 15: | Line 15: | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU35660 mnaA] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU35660 mnaA] | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/subtipathways/search.php?enzyme=MnaA MnaA]''' | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 42 kDa, 5.469 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 42 kDa, 5.469 | ||
Revision as of 11:43, 8 April 2014
- Description: UDP-N-acetylglucosamine 2-epimerase
| Gene name | mnaA |
| Synonyms | yvyH |
| Essential | yes PubMed |
| Product | UDP-N-acetylglucosamine 2-epimerase |
| Function | biosynthesis of teichoic acid |
| Gene expression levels in SubtiExpress: mnaA | |
| Metabolic function and regulation of this protein in SubtiPathways: MnaA | |
| MW, pI | 42 kDa, 5.469 |
| Gene length, protein length | 1140 bp, 380 aa |
| Immediate neighbours | tagU, gtaB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 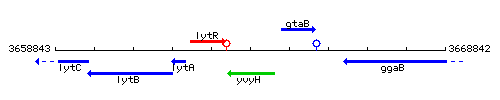
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35660
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU35660
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UDP-N-acetyl-D-glucosamine = UDP-N-acetyl-D-mannosamine (according to Swiss-Prot)
- Protein family: UDP-N-acetylglucosamine 2-epimerase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU35660
- UniProt: P39131
- KEGG entry: [2]
- E.C. number: 5.1.3.14
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Sheng-Chia Chen, Chi-Hung Huang, Chia Shin Yang, Jai-Shin Liu, Shu-Min Kuan, Yeh Chen
Crystal structures of the archaeal UDP-GlcNAc 2-epimerase from Methanocaldococcus jannaschii reveal a conformational change induced by UDP-GlcNAc.
Proteins: 2014, 82(7);1519-26
[PubMed:24470206]
[WorldCat.org]
[DOI]
(I p)
J Badger, J M Sauder, J M Adams, S Antonysamy, K Bain, M G Bergseid, S G Buchanan, M D Buchanan, Y Batiyenko, J A Christopher, S Emtage, A Eroshkina, I Feil, E B Furlong, K S Gajiwala, X Gao, D He, J Hendle, A Huber, K Hoda, P Kearins, C Kissinger, B Laubert, H A Lewis, J Lin, K Loomis, D Lorimer, G Louie, M Maletic, C D Marsh, I Miller, J Molinari, H J Muller-Dieckmann, J M Newman, B W Noland, B Pagarigan, F Park, T S Peat, K W Post, S Radojicic, A Ramos, R Romero, M E Rutter, W E Sanderson, K D Schwinn, J Tresser, J Winhoven, T A Wright, L Wu, J Xu, T J R Harris
Structural analysis of a set of proteins resulting from a bacterial genomics project.
Proteins: 2005, 60(4);787-96
[PubMed:16021622]
[WorldCat.org]
[DOI]
(I p)
Blazenka Soldo, Vladimir Lazarevic, Harold M Pooley, Dimitri Karamata
Characterization of a Bacillus subtilis thermosensitive teichoic acid-deficient mutant: gene mnaA (yvyH) encodes the UDP-N-acetylglucosamine 2-epimerase.
J Bacteriol: 2002, 184(15);4316-20
[PubMed:12107153]
[WorldCat.org]
[DOI]
(P p)