Difference between revisions of "YjbH"
(→Original Publications) |
|||
| Line 149: | Line 149: | ||
<pubmed> 23375660 23479438,19609260</pubmed> | <pubmed> 23375660 23479438,19609260</pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>17293416, 19074380 17908206, 20525796 21378193,21947404 24417481</pubmed> | + | <pubmed>17293416, 19074380 17908206, 20525796 21378193,21947404 24417481 24942655 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:51, 20 June 2014
- Description: adaptor protein for ClpX-ClpP-catalyzed Spx degradation, confers resistance against nitrosating agents
| Gene name | yjbH |
| Synonyms | |
| Essential | no |
| Product | adaptor protein |
| Function | stimulation of Spx degradation |
| Gene expression levels in SubtiExpress: yjbH | |
| Interactions involving this protein in SubtInteract: YjbH | |
| MW, pI | 31 kDa, 5.206 |
| Gene length, protein length | 825 bp, 275 aa |
| Immediate neighbours | yizD, yjbI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed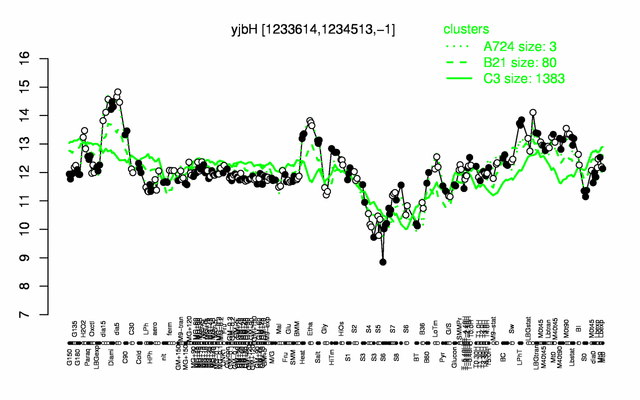
| |
Contents
Categories containing this gene/protein
proteolysis, resistance against other toxic compounds (nitric oxide, phenolic acids, flavonoids, oxalate)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11550
Phenotypes of a mutant
- increased thermotolerance due to increased stabiliy of Spx and thus increased expression of trxA PubMed
Database entries
- BsubCyc: BSU11550
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: adaptor protein for ClpX-ClpP-catalyzed Spx degradation PubMed
- Protein family: UPF0413 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: cytosolic protein
Database entries
- BsubCyc: BSU11550
- Structure:
- UniProt: O31606
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Peter Zuber, Oregon Health and Science University, USA Homepage
Claes von Wachenfeldt, Lund University, Sweden Homepage
Your additional remarks
References
Reviews
Original Publications