Difference between revisions of "LytE"
(→Biological materials) |
|||
| Line 152: | Line 152: | ||
* '''two-hybrid system:''' | * '''two-hybrid system:''' | ||
| + | |||
| + | * '''FLAG-tag construct:''' | ||
| + | ** GP2020 ''lytE-3xFLAG spec'' (based on [[pGP1331]]), available in [[Jörg Stülke]]'s lab | ||
* '''Antibody:''' | * '''Antibody:''' | ||
Revision as of 13:41, 24 September 2014
- Description: cell wall hydrolase (major autolysin) for cell elongation and separation, D,L-endopeptidase-type autolysin
| Gene name | lytE |
| Synonyms | papQ, cwlF |
| Essential | no |
| Product | cell wall hydrolase (major autolysin),endopeptidase-type autolysin |
| Function | major autolysin, cell elongation and separation |
| Gene expression levels in SubtiExpress: lytE | |
| MW, pI | 37 kDa, 10.713 |
| Gene length, protein length | 1029 bp, 343 aa |
| Immediate neighbours | phoA, citR |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 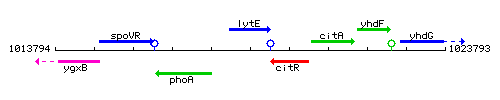
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed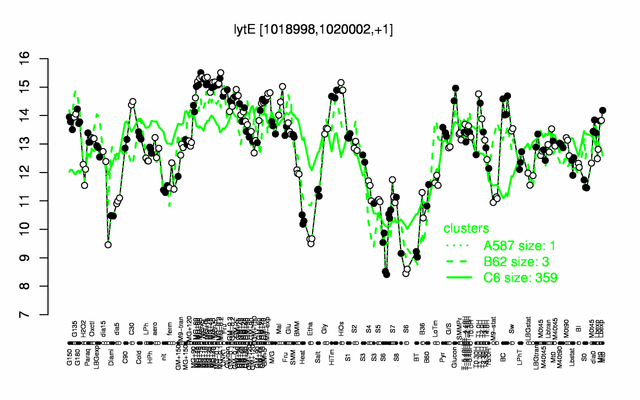
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover, cell wall synthesis
This gene is a member of the following regulons
SigH regulon, SigI regulon, Spo0A regulon, WalR regulon
The gene
Basic information
- Locus tag: BSU09420
Phenotypes of a mutant
- a cwlO lytE mutant is not viable PubMed
- growth defect at high temperature PubMed
- inactivation of lytE strongly restores beta-lactam resistance in a sigM mutant by delaying cell lysis PubMed
- a lytE mutation is synthetically lethal with ftsE and ftsX mutation (due to a lack of autolysin activity) PubMed
- a lytE mutation increases the cell separation defect of a lytF mutant PubMed
- cells are thinner (reduced diameter) as compared to the wild type PubMed
Database entries
- BsubCyc: BSU09420
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: nlpC/p60 family (according to Swiss-Prot)
- Paralogous protein(s): the C-terminal D,L-endopeptidase domains of LytE, LytF, CwlS, and CwlO exhibit strong sequence similarity
Extended information on the protein
- Kinetic information:
- Domains:
- contains three N-acetylglucosamine-polymer-binding LysM domains PubMed
- C-terminal D,L-endopeptidase domain PubMed
- Modification:
Database entries
- BsubCyc: BSU09420
- Structure:
- UniProt: P54421
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: lytE PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- FLAG-tag construct:
- GP2020 lytE-3xFLAG spec (based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Wan-Zhen Huang, Jyun-Jhih Wang, Hui-Ju Chen, Jung-Tze Chen, Gwo-Chyuan Shaw
The heat-inducible essential response regulator WalR positively regulates transcription of sigI, mreBH and lytE in Bacillus subtilis under heat stress.
Res Microbiol: 2013, 164(10);998-1008
[PubMed:24125693]
[WorldCat.org]
[DOI]
(I p)
Patricia Domínguez-Cuevas, Ida Porcelli, Richard A Daniel, Jeff Errington
Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis.
Mol Microbiol: 2013, 89(6);1084-98
[PubMed:23869552]
[WorldCat.org]
[DOI]
(I p)
Jeffrey Meisner, Paula Montero Llopis, Lok-To Sham, Ethan Garner, Thomas G Bernhardt, David Z Rudner
FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis.
Mol Microbiol: 2013, 89(6);1069-83
[PubMed:23855774]
[WorldCat.org]
[DOI]
(I p)
Letal I Salzberg, Leagh Powell, Karsten Hokamp, Eric Botella, David Noone, Kevin M Devine
The WalRK (YycFG) and σ(I) RsgI regulators cooperate to control CwlO and LytE expression in exponentially growing and stressed Bacillus subtilis cells.
Mol Microbiol: 2013, 87(1);180-95
[PubMed:23199363]
[WorldCat.org]
[DOI]
(I p)
Yun Luo, John D Helmann
Analysis of the role of Bacillus subtilis σ(M) in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis.
Mol Microbiol: 2012, 83(3);623-39
[PubMed:22211522]
[WorldCat.org]
[DOI]
(I p)
Masayuki Hashimoto, Seika Ooiwa, Junichi Sekiguchi
Synthetic lethality of the lytE cwlO genotype in Bacillus subtilis is caused by lack of D,L-endopeptidase activity at the lateral cell wall.
J Bacteriol: 2012, 194(4);796-803
[PubMed:22139507]
[WorldCat.org]
[DOI]
(I p)
Chi-Ling Tseng, Jung-Tze Chen, Ju-Hui Lin, Wan-Zhen Huang, Gwo-Chyuan Shaw
Genetic evidence for involvement of the alternative sigma factor SigI in controlling expression of the cell wall hydrolase gene lytE and contribution of LytE to heat survival of Bacillus subtilis.
Arch Microbiol: 2011, 193(9);677-85
[PubMed:21541672]
[WorldCat.org]
[DOI]
(I p)
Chyi-Liang Chen, Sau-Ching Wu, Wai Mui Tjia, Christopher L C Wang, Manfred J Lohka, Sui-Lam Wong
Development of a LytE-based high-density surface display system in Bacillus subtilis.
Microb Biotechnol: 2008, 1(2);177-90
[PubMed:21261835]
[WorldCat.org]
[DOI]
(I p)
Paola Bisicchia, David Noone, Efthimia Lioliou, Alistair Howell, Sarah Quigley, Thomas Jensen, Hanne Jarmer, Kevin M Devine
The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis.
Mol Microbiol: 2007, 65(1);180-200
[PubMed:17581128]
[WorldCat.org]
[DOI]
(P p)
Rut Carballido-López, Alex Formstone, Ying Li, S Dusko Ehrlich, Philippe Noirot, Jeff Errington
Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE.
Dev Cell: 2006, 11(3);399-409
[PubMed:16950129]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
Hiroki Yamamoto, Shin-ichirou Kurosawa, Junichi Sekiguchi
Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and stability of these enzymes to cell wall-bound or extracellular proteases.
J Bacteriol: 2003, 185(22);6666-77
[PubMed:14594841]
[WorldCat.org]
[DOI]
(P p)
R Ohnishi, S Ishikawa, J Sekiguchi
Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis.
J Bacteriol: 1999, 181(10);3178-84
[PubMed:10322020]
[WorldCat.org]
[DOI]
(P p)
S Ishikawa, Y Hara, R Ohnishi, J Sekiguchi
Regulation of a new cell wall hydrolase gene, cwlF, which affects cell separation in Bacillus subtilis.
J Bacteriol: 1998, 180(9);2549-55
[PubMed:9573210]
[WorldCat.org]
[DOI]
(P p)
P Margot, M Wahlen, A Gholamhoseinian, P Piggot, D Karamata
The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase.
J Bacteriol: 1998, 180(3);749-52
[PubMed:9457885]
[WorldCat.org]
[DOI]
(P p)