Difference between revisions of "BglS"
| Line 79: | Line 79: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 127: | Line 127: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' GP427 (licT-bglS, erm), BGW7 (cat), both available in the [[Stülke]] lab | + | * '''Mutant:''' GP427 (''[[licT]]-[[bglS]]'', erm), BGW7 (cat), both available in the [[Stülke]] lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 145: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed>18957862 12850135 6087283 8245830 8245831 8606172, 23459129 </pubmed> | + | <pubmed>18957862 12850135 6087283 8245830 8245831 8606172, 23459129 24391357 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:22, 7 January 2014
- Description: endo-beta-1,3-1,4 glucanase
| Gene name | bglS |
| Synonyms | bgl, licS |
| Essential | no |
| Product | endo-beta-1,3-1,4 glucanase |
| Function | lichenan degradation |
| Gene expression levels in SubtiExpress: bglS | |
| Metabolic function and regulation of this protein in SubtiPathways: bglS | |
| MW, pI | 27 kDa, 6.482 |
| Gene length, protein length | 726 bp, 242 aa |
| Immediate neighbours | citH, licT |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 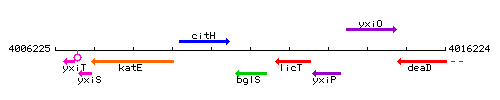
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed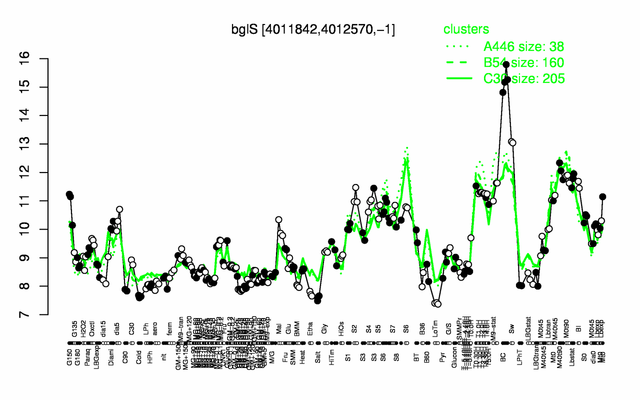
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU39070
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of (1->4)-beta-D-glucosidic linkages in beta-D-glucans containing (1->3)- and (1->4)-bonds (according to Swiss-Prot)
- Protein family: glycosyl hydrolase 16 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: extracellular (signal peptide) PubMed
Database entries
- Structure: 3O5S
- UniProt: P04957
- KEGG entry: [3]
- E.C. number: 3.2.1.73
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- CcpA: transcription repression PubMed
- LicT: binding to an RNA switch results in transcriptional antitermination PubMed
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References