Difference between revisions of "FabF"
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 69: | Line 65: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 87: | Line 82: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 153: | Line 148: | ||
<pubmed> 15952903 17919287</pubmed> | <pubmed> 15952903 17919287</pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | + | <pubmed>18384517, 12837788,12737802, 12682299 21383089 24641521 21542858,22178969</pubmed> | |
| − | <pubmed>18384517, 12837788,12737802, 12682299 21383089</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:34, 20 March 2014
- Description: beta-ketoacyl-acyl carrier protein synthase II, involved in the control of membrane fluidity
| Gene name | fabF |
| Synonyms | yjaY |
| Essential | yes PubMed |
| Product | beta-ketoacyl-acyl carrier protein synthase II |
| Function | fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fabF | |
| Metabolic function and regulation of this protein in SubtiPathways: fabF | |
| MW, pI | 43 kDa, 4.768 |
| Gene length, protein length | 1239 bp, 413 aa |
| Immediate neighbours | fabHA, yjaZ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 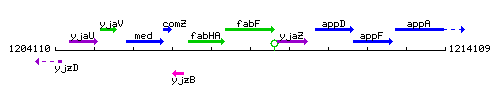
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
membrane dynamics, biosynthesis of lipids, cell envelope stress proteins (controlled by SigM, V, W, X, Y), essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11340
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: beta-ketoacyl-ACP synthases family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- UniProt: O34340
- KEGG entry: [3]
- E.C. number: 2.3.1.179
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications