Difference between revisions of "LytE"
(→Extended information on the protein) |
|||
| Line 59: | Line 59: | ||
* growth defect at high temperature {{PubMed|21541672}} | * growth defect at high temperature {{PubMed|21541672}} | ||
* inactivation of ''[[lytE]]'' strongly restores beta-lactam resistance in a ''[[sigM]]'' mutant by delaying cell lysis {{PubMed|22211522}} | * inactivation of ''[[lytE]]'' strongly restores beta-lactam resistance in a ''[[sigM]]'' mutant by delaying cell lysis {{PubMed|22211522}} | ||
| − | * a ''[[lytE]]'' mutation is synthetically lethal with ''[[ftsE]]'' and ''[[ftsX]]'' mutation (due to a lack of autolysin activity) {{PubMed|23855774}} | + | * a ''[[lytE]]'' mutation is synthetically lethal with ''[[ftsE]]'' and ''[[ftsX]]'' mutation (due to a lack of autolysin activity) {{PubMed|23869552,23855774}} |
* a ''[[lytE]]'' mutation increases the cell separation defect of a ''[[lytF]]'' mutant {{PubMed|23855774}} | * a ''[[lytE]]'' mutation increases the cell separation defect of a ''[[lytF]]'' mutant {{PubMed|23855774}} | ||
| − | + | * cells are thinner (reduced diameter) as compared to the wild type {{PubMed|23869552}} | |
=== Database entries === | === Database entries === | ||
| Line 85: | Line 85: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
** C-terminal D,L-endopeptidase domain {{PubMed|22139507}} | ** C-terminal D,L-endopeptidase domain {{PubMed|22139507}} | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | ** activity requires | + | ** activity requires functional [[MreB]] and [[MreBH]] {{PubMed|23869552,16950129}} |
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| Line 101: | Line 101: | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| − | |||
** binds the cell wall {{PubMed|21261835}} | ** binds the cell wall {{PubMed|21261835}} | ||
** localizes to cell septa, poles and lateral sidewall of the cell (via the N-terminal domain) {{PubMed|22139507}} | ** localizes to cell septa, poles and lateral sidewall of the cell (via the N-terminal domain) {{PubMed|22139507}} | ||
| + | ** localization to lateral cell wall depends on the interaction with [[MreBH]] {{PubMed|23869552}} | ||
=== Database entries === | === Database entries === | ||
| Line 159: | Line 159: | ||
<pubmed>23066944</pubmed> | <pubmed>23066944</pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>21261835,22211522 16950129,17581128,14594841,9457885,9573210,10322020, 20059685,14651647, 21541672 22139507 23855774</pubmed> | + | <pubmed>21261835,22211522 16950129,17581128,14594841,9457885,9573210,10322020, 20059685,14651647,23869552 21541672 22139507 23855774</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 19:35, 14 December 2013
- Description: cell wall hydrolase (major autolysin) for cell elongation and separation, D,L-endopeptidase-type autolysin
| Gene name | lytE |
| Synonyms | papQ, cwlF |
| Essential | no |
| Product | cell wall hydrolase (major autolysin),endopeptidase-type autolysin |
| Function | major autolysin, cell elongation and separation |
| Gene expression levels in SubtiExpress: lytE | |
| MW, pI | 37 kDa, 10.713 |
| Gene length, protein length | 1029 bp, 343 aa |
| Immediate neighbours | phoA, citR |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 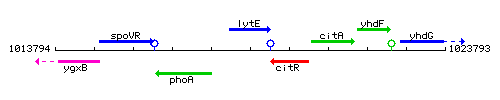
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover, cell wall synthesis
This gene is a member of the following regulons
SigH regulon, SigI regulon, Spo0A regulon, WalR regulon
The gene
Basic information
- Locus tag: BSU09420
Phenotypes of a mutant
- a cwlO lytE mutant is not viable PubMed
- growth defect at high temperature PubMed
- inactivation of lytE strongly restores beta-lactam resistance in a sigM mutant by delaying cell lysis PubMed
- a lytE mutation is synthetically lethal with ftsE and ftsX mutation (due to a lack of autolysin activity) PubMed
- a lytE mutation increases the cell separation defect of a lytF mutant PubMed
- cells are thinner (reduced diameter) as compared to the wild type PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: nlpC/p60 family (according to Swiss-Prot)
- Paralogous protein(s): the C-terminal D,L-endopeptidase domains of LytE, LytF, CwlS, and CwlO exhibit strong sequence similarity
Extended information on the protein
- Kinetic information:
- Modification:
Database entries
- Structure:
- UniProt: P54421
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: lytE PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications