Difference between revisions of "OxdD"
(→Expression and regulation) |
|||
| Line 60: | Line 60: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 78: | Line 75: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 139: | Line 136: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed>20464388 </pubmed> | + | <pubmed>20464388 23202530</pubmed> |
==Original publications== | ==Original publications== | ||
<pubmed> 11546787 15699190,14973022 20601499 22171814</pubmed> | <pubmed> 11546787 15699190,14973022 20601499 22171814</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:38, 4 January 2014
- Description: oxalate decarboxylase, inner spore coat protein
| Gene name | oxdD |
| Synonyms | yoaN |
| Essential | no |
| Product | oxalate decarboxylase |
| Function | protection of the spore |
| Gene expression levels in SubtiExpress: oxdD | |
| MW, pI | 43 kDa, 5.359 |
| Gene length, protein length | 1176 bp, 392 aa |
| Immediate neighbours | yozS, yoaO |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed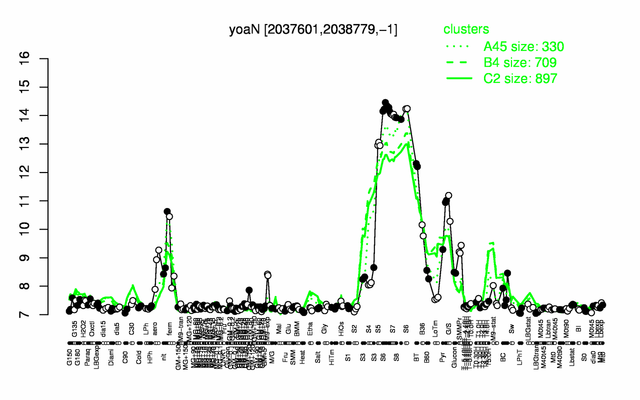
| |
Contents
Categories containing this gene/protein
sporulation proteins, resistance against other toxic compounds (nitric oxide, phenolic acids, flavonoids, oxalate)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU18670
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Oxalate = formate + CO2 (according to Swiss-Prot)
- Protein family: UPF0361 family (according to Swiss-Prot)
- Paralogous protein(s): OxdC
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O34767
- KEGG entry: [3]
- E.C. number: 4.1.1.2
Additional information
Expression and regulation
- Operon: oxdD PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Peter T McKenney, Adam Driks, Patrick Eichenberger
The Bacillus subtilis endospore: assembly and functions of the multilayered coat.
Nat Rev Microbiol: 2013, 11(1);33-44
[PubMed:23202530]
[WorldCat.org]
[DOI]
(I p)
Miia R Mäkelä, Kristiina Hildén, Taina K Lundell
Oxalate decarboxylase: biotechnological update and prevalence of the enzyme in filamentous fungi.
Appl Microbiol Biotechnol: 2010, 87(3);801-14
[PubMed:20464388]
[WorldCat.org]
[DOI]
(I p)
Original publications
Peter T McKenney, Patrick Eichenberger
Dynamics of spore coat morphogenesis in Bacillus subtilis.
Mol Microbiol: 2012, 83(2);245-60
[PubMed:22171814]
[WorldCat.org]
[DOI]
(I p)
Sébastien Potot, Cláudia R Serra, Adriano O Henriques, Ghislain Schyns
Display of recombinant proteins on Bacillus subtilis spores, using a coat-associated enzyme as the carrier.
Appl Environ Microbiol: 2010, 76(17);5926-33
[PubMed:20601499]
[WorldCat.org]
[DOI]
(I p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
Teresa Costa, Leif Steil, Lígia O Martins, Uwe Völker, Adriano O Henriques
Assembly of an oxalate decarboxylase produced under sigmaK control into the Bacillus subtilis spore coat.
J Bacteriol: 2004, 186(5);1462-74
[PubMed:14973022]
[WorldCat.org]
[DOI]
(P p)
A Tanner, L Bowater, S A Fairhurst, S Bornemann
Oxalate decarboxylase requires manganese and dioxygen for activity. Overexpression and characterization of Bacillus subtilis YvrK and YoaN.
J Biol Chem: 2001, 276(47);43627-34
[PubMed:11546787]
[WorldCat.org]
[DOI]
(P p)